Abstract
Background and Objectives
We evaluated the symptom improvement, surgical outcomes and post-operative complications of the figure of 8 anchoring suture technique using polycaprolactone (PCL) nasal mesh for the treatment of caudal septal subluxation.
Subjects and Method
We conducted a retrospective study of patients who underwent between March 2020 and March 2021 endonasal septoplasty using the figure of 8 anchoring suture technique and a PCL nasal mesh as a protective supporting graft. Fourteen patients were divided into two groups, the allergic and non-allergic rhinitis group. Symptom improvements were assessed using the Nasal Obstruction Symptoms Evaluation (NOSE) scores and visual analog scale (VAS) scores for epistaxis and headache. Post-operative patient-reported subjective changes in nasal obstruction were also recorded. All evaluations were conducted one to four months post-operatively.
Results
Post-operative endoscopic examination revealed that all patients had their septum straightened. The mean post-operative NOSE scores in overall and each item were significantly lower than the mean NOSE scores in the pre-operative period (p<0.05). The mean post-operative NOSE scores for patients both with and without allergic rhinitis were also significantly decreased compared to those in the pre-operative period (p<0.05). A decrease in VAS scores was significant (p=0.008) for headache but not for epistaxis (p=0.141). All patients reported improvement of subjective nasal obstruction post-operatively.
Nasal septal deviation is common cause of nasal obstruction, and even small caudal septal deviations can lead to severe symptoms by narrowing the external valve area. Correction of caudal septal deviation is challenging owing to intrinsic cartilage memory and the possibility of weakening of the caudal septal support with subsequent nasal deformity [1]. In particular, the caudal part of the septum abuts the anterior nasal spine and premaxilla bone, and its subluxation or dislocation can further aggravate nasal congestion and stuffiness.
A previous study introduced the figure of 8 anchoring suture technique to correct caudal subluxation over the anterior nasal spine [2]; however, a fragile or weakened manipulated cartilaginous caudal septum can be fractured due to the strong compression force generated by the knot of an anchoring suture, causing subsequent nasal deformity. Therefore, several surgeons have started to use autologous implants, such as nasal septal cartilages or bony septum, as grafts to protect and further support the caudal septal cartilage [3-5]. However, surgeons may not always obtain straight and sufficiently strong autologous implants in cases of severely deviated septal cartilage, trauma, and revision septal surgery. Moreover, bone resorption can cause recurrence of deviation, and the bony septum can further narrow the nasal airway where it is placed [6].
Several alloplastic implant materials have been developed to overcome the disadvantages of autologous implants; however, complications such as infection, displacement, protrusion, skin problems, and contracture are still an issue [7,8]. Recently, a three-dimensional (3D) printed polycaprolactone (PCL) nasal mesh has been introduced as an alternative to autologous implants. It is a biocompatible and biodegradable nasal implant, proven to have proper mechanical support, thinness, and surgical manipulability [9-11].
This study aimed to evaluate the symptom improvement, surgical outcomes, and post-operative complications in patients with caudal septal subluxation treated by the figure of 8 anchoring suture technique using a PCL nasal mesh as a protective and supportive material for the manipulated caudal septal cartilage.
Fourteen patients with caudal septal subluxation who underwent endonasal septoplasty with the figure of 8 anchoring suture technique and a PCL nasal mesh (TnR Mesh; T&R Biofab Co. Ltd, Siheung, Korea) as a structurally supporting graft between March 2020 and March 2021 at the Korea University Guro Hospital were included. Information on the patient’s demographic characteristics; history of nasal surgery; comorbid diseases (chronic rhinosinusitis, asthma, and allergic rhinitis); and the degree of symptom improvement were gathered from medical records. All patients complained of nasal obstruction for more than three months pre-operatively. In particular, patients with allergic rhinitis were those who continued to have persistent nasal obstruction despite using nasal decongestants and antihistamines. A single surgeon (I.H.P) performed all surgical procedures and patients with concomitant endoscopic sinus surgery, rhinoplasty, excision, or submucosal turbinoplasty were included in this study. Patients with allergic rhinitis continued using nasal decongestants and antihistamines post-operatively. We divided the patients into those with and without allergic rhinitis to perform a subanalysis. The Institutional Review Board (IRB) of Korea University Guro Hospital approved this study (IRB No. 2021GR0258).
All surgical procedures were performed under general anesthesia. A hemitransfixion incision was made at the left caudal end of the septal cartilage through the endonasal approach, and the bilateral mucoperichondrial flap was raised from the caudal end to the perpendicular plate of the ethmoid, vomer and maxillary crest (Figs. 1A and 2A). Deviated cartilaginous and bony portions were excised. The caudal septal cartilage was separated from the anterior nasal spine for repositioning and the excessive part of the caudal septal cartilage over the anterior nasal spine was excised in a v-shaped notch (Figs. 1B and 2B). Thereafter, two PCL nasal meshes were carved to oval to round shape with a diameter of 5-8 mm and sutured on both sides of the caudal septal cartilage using two to three stitches (4-0 polydioxanone sutures) (Fig. 1C). Two holes were drilled through the anterior nasal spine (Fig. 2C). The complex of caudal septal cartilage with PCL nasal meshes was realigned along the midline and fixed against the anterior nasal spine by the figure of 8 anchoring suture technique through the holes of the anterior nasal spine with nylon 3-0 (Figs. 1D and 2D). The hemitransfixion incision was closed using a 3-0 vicryl suture. Silastic sheets were positioned bilaterally in the nasal cavity and sutured to the septum. Bilateral nasal packing was performed (RAPID RHINO; Arthocare, Austin, TX, USA), and the operation was completed once straightening of the deviated caudal septum was confirmed. Bilateral nasal packing was removed on the second day, and the silastic sheets were removed 2 weeks after the operation.
Two otolaryngologists who were blinded to the study individually evaluated the pre- and post-operative endoscopic photographs of the caudal septum and categorized them into three groups of “straight,” “improved but with residual deviation,” and “no change.” If there is a disagreement between two otolaryngologists, the worse outcome was recorded. Symptom improvement was evaluated using Nasal Obstruction Symptoms Evaluation (NOSE), and it constituted of five individual self-rated items scored from 0 (no problem) to 20 (extremely severe) per item. Visual analog scale (VAS) (scores ranging from 0 [no problem] to 10 [extremely severe]) was used for assessing epistaxis and headache. Additionally, we ascertained subjective surgical outcomes via a patient-reported questionnaire survey that enquired about a subjective change of nasal obstruction (“considerably worsened,” “worsened,” “no change,” “improved,” and “considerably improved”) post-operatively. Patients evaluated pre- and post-operative symptom scores at an outpatient clinic, and all post-operative evaluations including endoscopic photographs were performed from 1 to 4 months after surgery.
Statistical analysis was performed using the SPSS software (version 22.0, IBM Corp., Armonk, NY, USA). For quantitative variables, the values were expressed as mean (standard deviation). A paired t-test and Wilcoxon signed-rank test were used to compare pre- and post-operative NOSE and VAS scores. For the subanalysis, a comparison of pre and postoperative NOSE scores in patients with and without allergic rhinitis was conducted using Wilcoxon signed-rank test. Statistical significance was set at p<0.05.
A total of 14 patients (11 male and 3 female) were included in this study, and their mean age was 40.6±17.3 years (range, 17-72 years). Three (21.4%), one (7.1%), and six (42.8%) patients had chronic rhinosinusitis, asthma, and allergic rhinitis as comorbid diseases, respectively. None of the patients had a history of previous septal surgeries. Three (21.4%), eleven (78.6%), one (7.1%), and one (7.1%) patients underwent endoscopic sinus surgery, turbinate surgery, intranasal excision, and rhinoplasty at the same time as their endonasal septoplasty, respectively. The mean follow-up period was 63.6±21.1 days (range, 30-124 days).
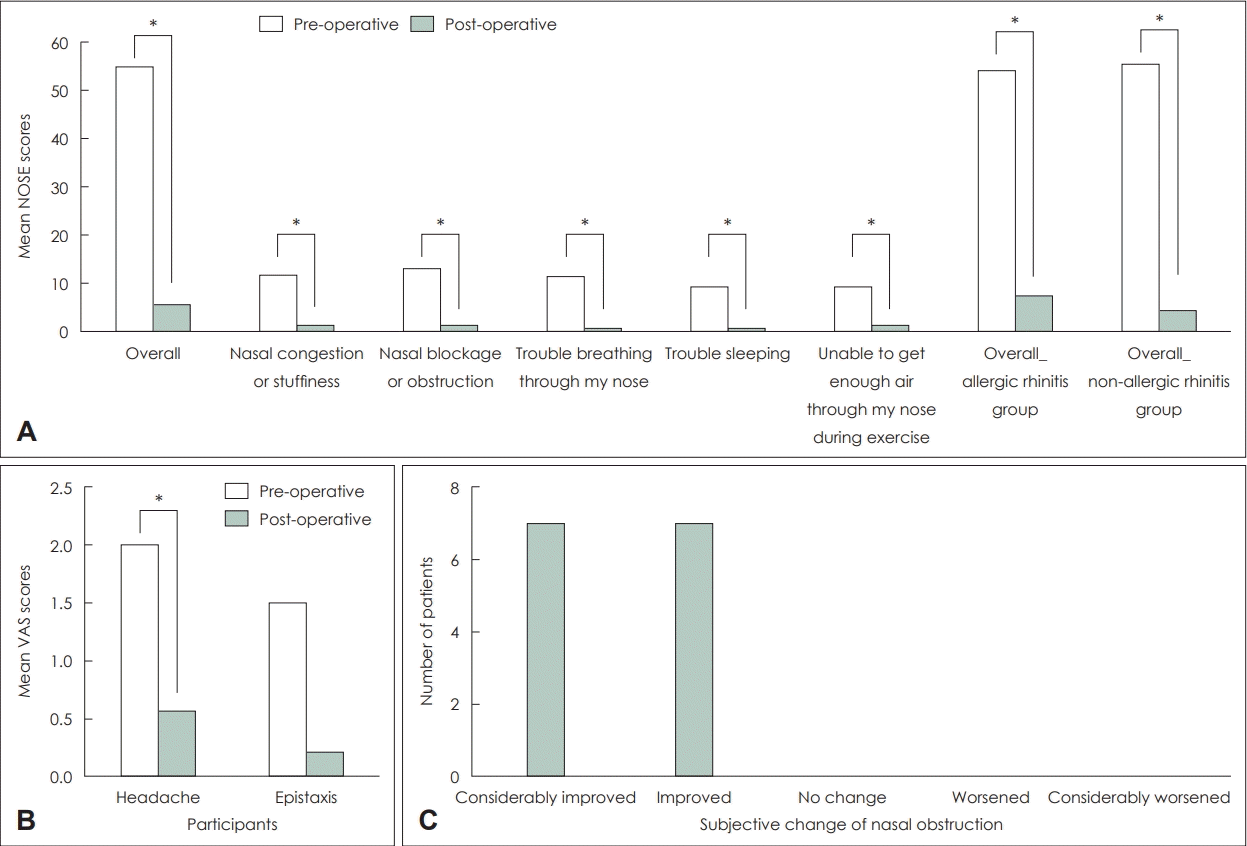
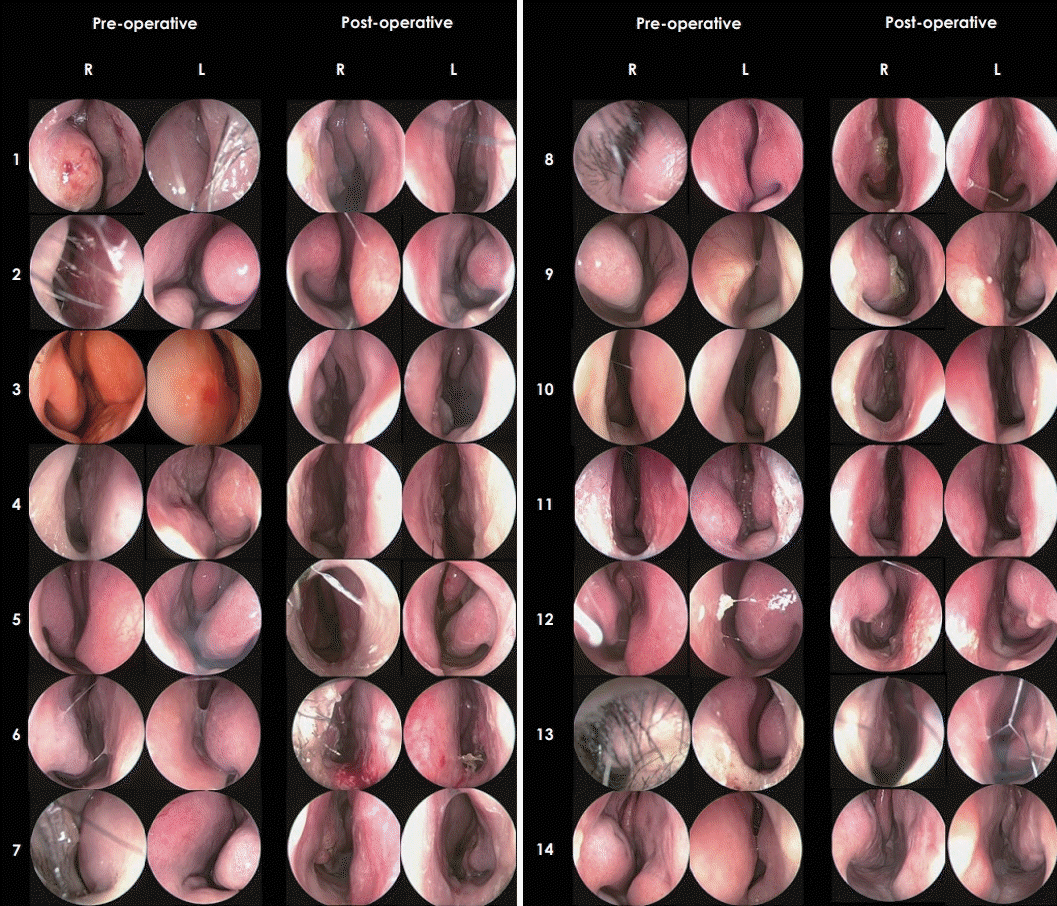
Pre- and post-operative endoscopic images of the bilateral nasal cavity in all 14 patients are shown in Fig. 3. Post-operative endoscopic examinations revealed that all patients had their septum straightened. The mean post-operative NOSE scores in overall and each item were significantly lower than the mean NOSE scores in the pre-operative period (Fig. 4A). The mean pre- and post-operative NOSE scores were 55.2± 23.8 and 5.7±11.4, respectively (p<0.05). The mean post-operative NOSE scores for both patients with and without allergic rhinitis were also significantly decreased compared to those in the pre-operative period (Fig. 4A). The mean pre- and post-operative NOSE scores were 54.1±24.1 and 7.5±16.0 for patients with allergic rhinitis, respectively (p<0.001), and 55.6± 25.2 and 4.3±7.2 for patients without allergic rhinitis, respectively (p=0.002). A decrease in VAS scores was statistically significant (p=0.008) for headache but not for epistaxis (p= 0.141). The VAS score of headache decreased from 2.0 to 0.6 post-operatively (Fig. 4B). In terms of subjective surgical outcomes, every patient reported that their nasal obstruction had improved or considerably improved post-operatively (Fig. 4C).
Two post-operative complications were observed. A male patient visited the outpatient clinic with septal epistaxis 7 days after surgery and was managed using electrocautery. Another male patient (No. 6 in Fig. 3, endoscopic photographs taken before extrusion) visited the outpatient clinic with extrusion of the implanted PCL nasal mesh and foreign body sensation in the left nasal cavity 4 months after surgery. The nasal mesh was easily removed, and there were no signs of infection or inflammation. Moreover, NOSE scores did not increase again, and the caudal septum remained straight in position. NOSE scores obtained 4 months after surgery, which was after removal of extruded nasal mesh, were included for statistical analysis.
Several septoplasty techniques including swinging door maneuvers, cartilage reshaping, and repositioning methods have been proposed to correct caudal septal cartilage subluxation [2,12-14]. However, none of them are widely accepted as the standard procedure owing to the possibility of undercorrection or overcorrection of caudal cartilage deviation. Moreover, weakening of the L-strut structure with subsequent nasal tip deformity is not ignorable, and it makes surgeons passive and cautious in correcting caudal septal subluxation. A previous study published in 2005 introduced the figure of 8 anchoring suture technique to correct caudal subluxation over the anterior nasal spine [2], and this surgical technique has been adopted by several surgeons pursuing radical management of caudal septal subluxation. However, a fragile or weakened manipulated cartilaginous caudal septum can be fractured due to the strong compression force generated by the knot of an anchoring suture, causing a subsequent nasal deformity. Tight suturing with sufficient force is essential for maintaining the shape or stability of the manipulated caudal septum.
Placement of grafts on the caudal septum is an effective method for protecting and supporting the manipulated caudal septum. Septal, auricular, and costal cartilages are possible autologous candidates as they are biocompatible, nonimmunogenic, and have a low risk of infection and extrusion [15-17]. Among these, septal bone is one of the most commonly harvested materials during surgery and has several advantages, including providing mechanical nasal tip support of the L-strut, preventing cartilage recoiling, and protecting the septal cartilage from being fractured due to the strong compression force generated by the knot of an anchoring suture. However, bony grafts have several disadvantages. First, it can be difficult to consistently harvest autologous septal bone with sufficient size and strength in cases of a severely deviated septum. Moreover, additional manipulation or thinning is required if the harvested bone is sufficiently thick to further narrow the nasal valve area. Therefore, ideal alloplastic grafting materials with sufficient strength but thin structures have been widely investigated.
Previous studies have reported the use of alloplastic bioabsorbable plates for an internal septal splint or L-strut support in septorhinoplasty and demonstrated good surgical outcomes for stabilizing and straightening the septum [18,19]. Particularly, a few studies of clinical application of 3D-printed PCL mesh in rhinoplasty proved its safe and effective aspects in terms of nasal reconstruction 11,20]. However, a limited number of studies have investigated endonasal septoplasty using a PCL implants for correcting caudal septal deviation. Recently, Kim, et al. [9] studied 20 patients with caudal septal deviations who underwent endonasal septoplasty using microporous PCL nasal implants as septal batten grafts, and showed good surgical results and a substantial decrease in post-operative NOSE scores. This study by Kim, et al. differed from our study in that they simply placed a PCL nasal mesh on the concave side. A PCL nasal mesh has not only the same advantages as bony grafts but also several additional merits. First, it is a commercially available material and its use is not affected by the severity of the septal deviation. Second, it is much easier to surgically manipulate than bony grafts, thereby reducing the operation time. For example, simple scissors are sufficient to tailor a PCL nasal mesh, while a bony graft requires drilling or surgical instruments. Third, a PCL nasal mesh is quite thin (0.5 mm) and, therefore, does not cause narrowing of the nasal valve area. Fourth, a PCL nasal mesh offers mechanical stability and protects the septal cartilage until the manipulated cartilage heals despite its small diameter. Its microporous structure is believed to enable ingrowth of host tissue and significant integration with the surrounding soft tissue without inflammation or immune response [20]. In addition, its microporous structure of a pore size of 500 μm enables vascular formation and growth. Generally, a pore size of 150-500 μm is known to be sufficient for vascularization and blood vessel invasion [21]. Lastly, a PCL is known to be degraded slowly resulting in lesser inflammatory or immune response and its structural stability can be maintained up to 2 to 4 years [20,22,23].
In this study, endonasal septoplasty using 3D printed PCL nasal meshes as protective and supportive grafts was performed for correcting caudal septal subluxation, and clinical outcomes were evaluated based on endoscopic images and questionnaires completed by patients. We achieved straightening and strengthening of the caudal septum in all 14 patients post-operatively, even with a small sized PCL nasal mesh (5-8 mm) (Fig. 3). This means that this surgical technique is reproducible and effectively protects the manipulated septal cartilage in addition to providing substantial mechanical support to the caudal septum. Furthermore, placement of PCL nasal meshes in a “sandwich” manner did not manifest further nasal obstruction, meaning that it did not significantly reduce the volume of the anterior nasal cavity. Substantial improvements in post-operative NOSE scores were observed, and every patient reported their nasal obstruction being “improved” or “considerably improved” after surgery. Even patients with allergic rhinitis, whose nasal congestion did not improve despite taking medications, benefited from the endonasal septoplasty with the figure of 8 anchoring suture technique and a PCL nasal mesh. For headache and epistaxis, there were not much differences in between pre- and post-operative VAS scores although a decrease in VAS scores for headache was statistically significant. Headache and epistaxis are nonspecific nasal symptoms, and initial VAS scores for epistaxis and headache were low. In this study, patients who underwent concurrent surgery (endoscopic sinus surgery, rhinoplasty or inferior turbinoplasty) were included. However, both endoscopic sinus surgery and rhinoplasty were considered to be irrelevant to nasal obstruction for the purpose of performing surgery because any distinct cause for nasal obstruction in nasal cavity was not found. Only cases in which nasal septal deviation was considered to be the sole cause of nasal obstruction were included. In addition, the purpose of concurrent turbinoplasty was that hypertrophy of inferior turbinate on the concave side could cause secondary nasal obstruction on the concave side that was rather narrowed as the deviated nasal septum was corrected. Therefore, it was not performed for the purpose of relieving the initial nasal obstruction.
Two complications were observed during the post-operative period, one of which was an extrusion of the implanted PCL nasal mesh and subsequent foreign body sensation in the left nasal cavity 4 months after surgery. Complications including infection, displacement, extrusion, and skin problems, related to alloplastic materials have been an issue in replacing autologous materials [7]. According to previous studies focusing on the safety and efficacy of using Gore-Tex in nasal dorsum surgery, the infection rates ranged from 0% to 3.2% [24-26]. Extrusion rates of silicone implants ranged from 2.1% to 3.7% [27]. In case of PCL, a preclinical study was performed in an experimental rabbit model and showed no post-operative complications such as graft extrusion and skin infection during the post-operative 12 weeks [28]. Further histologic assessment proved that inflammatory cell infiltration in the implanted groups was comparable to that in the sham group. A clinical study of using PCL in rhinoplasty was also performed and demonstrated that 101 patients whose follow-up period ranged from 12 to 30 months showed no definite graft warping, absorption, or extrusion [20]. Histopathological analysis found that the scaffold structure of implanted PCL preserved well during the post-operative 20 months and material-related inflammatory cell infiltration was not detected. Mesh-related infection occurred in two (2.0%) cases without extrusion. A similar study performing rhinoplasty with PCL mesh as a graft reported approximately 1% of material-related complication rates including mesh infection and exposure, and its mean followup period was 11.9 months [11]. In our study, infectious signs were not found in any of the 14 patients, including a male patient with nasal mesh extrusion. When the follow-up period was set to the date of the most recent outpatient visit, there was no further signs of infection or extrusion of the implanted PCL nasal mesh. The range of the follow-up period till the most recent outpatient visit was from 58 to 426 days and the mean follow-up period was 232.4 days. Therefore, results of this study were consistent with other studies utilizing a PCL nasal mesh, implying that a PCL nasal mesh is a safe and biocompatible material. However, there are several steps to take precautions against mesh infection or extrusion. First, the minimum amount of implant, but large enough to protect septal cartilage, should be used and the area where the septal incision and the implant meet should be minimized. Second, septal mucosa should not be tensioned by the implanted material. After surgery, frequent nasal irrigation is recommended and patients are advised not to irritate the surgical site by fingers.
This study had some limitations. First, there were a limited number of study patients. Second, the follow-up time was limited to 4 months after surgery; therefore, long-term outcomes were not assessed. Third, we included patients who underwent concomitant surgery (endoscopic sinus surgery, rhinoplasty or inferior turbinoplasty) with septoplasty, thereby increasing the heterogeneity of the study population. In other words, there are limitations in evaluating the effects of septoplasty and inferior turbinoplasty individually at the same time. Fourth, objective tools such as acoustic rhinometry were not utilized. Therefore, it was not possible to determine how a PCL nasal mesh affects the change in the volume of the nasal cavity.
Correction of caudal septal subluxation using the figure of 8 anchoring suture technique with a PCL nasal mesh as a protective and supportive graft was proven to be a successful method. A PCL nasal mesh effectively protected the manipulated septal cartilage from crumbling and allowed surgeons to use sufficient force when trying a figure of 8 knot. A PCL nasal mesh attached to the septal cartilage and the anterior nasal spine facilitated surgical correction of severe caudal septal subluxation, and exhibited mechanical stability, despite its small size until the manipulated cartilage was healed. Future large-scale studies with long-term follow-up data are needed to further clarify surgical and patient-reported outcomes in addition to the types and rates of post-operative complications.
Notes
Author Contribution
Conceptualization: Seok-Youl Choi, Jee Won Moon. Data curation: Seok-Youl Choi, Jee Won Moon. Formal analysis: Seok-Youl Choi, Su-Jong Kim. Investigation: Su-Jong Kim, Jae-Min Shin. Methodology: Seok-Youl Choi, Jee Won Moon. Project administration: Jae-Min Shin, Il-Ho Park. Software: Seok-Youl Choi. Supervision: Il-Ho Park. Validation: Su-Jong Kim, Jae-Min Shin, Il-Ho Park. Visualization: Seok-Youl Choi. Writing—original draft: Seok-Youl Choi. Writing—review & editing: Seok-Youl Choi, Il-Ho Park.
REFERENCES
1. Lee BJ, Chung YS, Jang YJ. Overcorrected septum as a complication of septoplasty. Am J Rhinol. 2004; 18(6):393–6.
2. Dhong HJ, Kim HY, Chung MK. Septoplasty with conservative resection and figure of 8 anchoring suture for the caudal septal deviation. Korean J Otorhinolaryngol-Head Neck Surg. 2005; 48(1):51–5.
3. Aksakal C. Surgical outcomes of bony batten grafting through endonasal septoplasty in the correction of caudal septum deviation. J Craniofac Surg. 2020; 31(1):162–5.
4. Chung YS, Seol JH, Choi JM, Shin DH, Kim YW, Cho JH, et al. How to resolve the caudal septal deviation? Clinical outcomes after septoplasty with bony batten grafting. Laryngoscope. 2014; 124(8):1771–6.
5. Wee JH, Lee JE, Cho SW, Jin HR. Septal batten graft to correct cartilaginous deformities in endonasal septoplasty. Arch Otolaryngol Head Neck Surg. 2012; 138(5):457–61.
6. Metzinger SE, Boyce RG, Rigby PL, Joseph JJ, Anderson JR. Ethmoid bone sandwich grafting for caudal septal defects. Arch Otolaryngol Head Neck Surg. 1994; 120(10):1121–5.
7. Kim HS, Park SS, Kim MH, Kim MS, Kim SK, Lee KC. Problems associated with alloplastic materials in rhinoplasty. Yonsei Med J. 2014; 55(6):1617–23.
8. Sajjadian A, Rubinstein R, Naghshineh N. Current status of grafts and implants in rhinoplasty: Part I. Autologous grafts. Plast Reconstr Surg. 2010; 125(2):40. e-9.
9. Kim DH, Yun WS, Shim JH, Park KH, Choi D, Park MI, et al. Clinical application of 3-dimensional printing technology for patients with nasal septal deformities: A multicenter study. JAMA Otolaryngol Head Neck Surg. 2018; 144(12):1145–52.
10. Ahn TH, Heo CY, Ahn KC. A compound osteocartilaginous graft with polycaprolactone (PCL) mesh in Asian rhinoplasty. J Plast Reconstr Aesthet Surg. 2020; 73(9):e6–7.
11. Hyun S, Woo S, Baek RM. Polycaprolactone mesh for asian rhinoplasty: Outcomes and complications of composite septal extension graft compared to mesh-only graft. Facial Plast Surg. In press 2021.
12. Akduman D, Haksever M, Yanilmaz M. Repositioning of the caudal septal dislocations with notching and suturing the cartilage to the nasal spine. Eur Arch Otorhinolaryngol. 2014; 271(1):81–5.
13. Pastorek NJ, Becker DG. Treating the caudal septal deflection. Arch Facial Plast Surg. 2000; 2(3):217–20.
14. Park JS, Lee YJ, Jun YJ, Lee JY, Baek BJ. Correction of caudal septal dislocation or subluxation with excision and suturing the cartilage to the premaxilla bone. Korean J Otorhinolaryngol-Head Neck Surg. 2014; 57(12):836–40.
15. Dingman RO. Correction of nasal deformities due to defects of the septum. Plast Reconstr Surg (1946). 1956; 18(4):291–304.
16. Jang YJ, Kim JM, Yeo NK, Yoo JH. Use of nasal septal bone to straighten deviated septal cartilage in correction of deviated nose. Ann Otol Rhinol Laryngol. 2009; 118(7):488–94.
17. Kim SA, Jang YJ. Caudal septal division and interposition batten graft: A novel technique to correct caudal septal deviation in septoplasty. Ann Otol Rhinol Laryngol. 2019; 128(12):1158–64.
18. Kim JN, Choi HG, Kim SH, Park HJ, Shin DH, Jo DI, et al. The efficacy of bioabsorbable mesh as an internal splint in primary septoplasty. Arch Plast Surg. 2012; 39(5):561–4.
19. Fuller JC, Levesque PA, Lindsay RW. Polydioxanone plates are safe and effective for L-strut support in functional septorhinoplasty. Laryngoscope. 2017; 127(12):2725–30.
20. Park YJ, Cha JH, Bang SI, Kim SY. Clinical application of three-dimensionally printed biomaterial polycaprolactone (PCL) in augmentation rhinoplasty. Aesthetic Plast Surg. 2019; 43(2):437–46.
21. Liu Y, Lim J, Teoh SH. Review: Development of clinically relevant scaffolds for vascularised bone tissue engineering. Biotechnol Adv. 2013; 31(5):688–705.
22. Sun H, Mei L, Song C, Cui X, Wang P. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials. 2006; 27(9):1735–40.
23. Azimi B, Nourpanah P, Rabiee M, Arbab S. Poly (ε-caprolactone) fiber: An overview. J Eng Fibers Fabr. 2014; 9(3):74–90.
24. Conrad K, Torgerson CS, Gillman GS. Applications of Gore-Tex implants in rhinoplasty reexamined after 17 years. Arch Facial Plast Surg. 2008; 10(4):224–31.
25. Jin HR, Lee JY, Yeon JY, Rhee CS. A multicenter evaluation of the safety of Gore-Tex as an implant in Asian rhinoplasty. Am J Rhinol. 2006; 20(6):615–9.
26. Owsley TG, Taylor CO. The use of Gore-Tex for nasal augmentation: A retrospective analysis of 106 patients. Plast Reconstr Surg. 1994; 94(2):241–8. discussion 249-50.
27. Malone M, Pearlman S. Dorsal augmentation in rhinoplasty: A survey and review. Facial Plast Surg. 2015; 31(3):289–94.
28. Park SH, Yun BG, Won JY, Yun WS, Shim JH, Lim MH, et al. New application of three-dimensional printing biomaterial in nasal reconstruction. Laryngoscope. 2017; 127(5):1036–43.
Fig. 1.
Schematic representation of caudal septal subluxation (A); excision of excessive septal cartilage (B); placement of PCL nasal meshes (C); fixation using a figure of 8 anchoring suture (D). PCL, polycaprolactone.
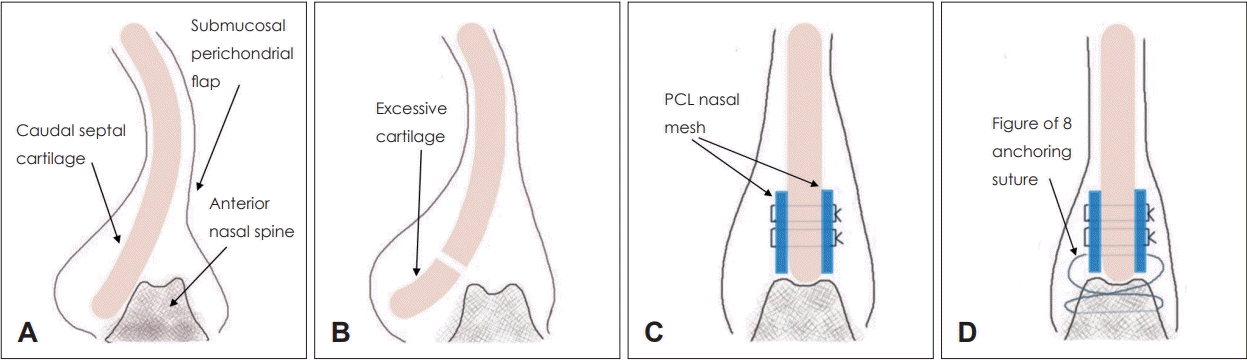
Fig. 2.
Intraoperative images of caudal septal subluxation (A); excision of excessive septal cartilage (B); two holes made through the anterior nasal spine by drilling (C); fixation using a figure of 8 anchoring suture (D).
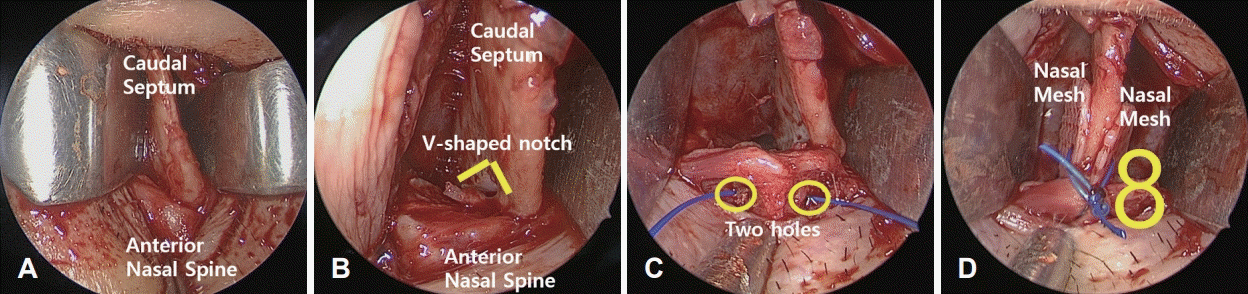
Fig. 4.
Evaluation of clinical outcomes. A: Pre- and postoperative- NOSE scores. B: Pre- and post-operative visual analog scores for headache and epistaxis. C: The post-operative subjective change of nasal obstruction. *statistically significant (p<0.05). NOSE, Nasal Obstruction Symptom Evaluation; VAS, visual analog scale.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download