Abstract
Tinnitus is defined as the conscious awareness of a sound without an identifiable external sound source, and tinnitus disorder as tinnitus with associated suffering. Chronic tinnitus has been anatomically and phenomenologically separated into three pathways: a lateral “sound” pathway, a medial “suffering” pathway, and a descending noise-canceling pathway. Here, the triple network model is proposed as a unifying framework common to neuropsychiatric disorders. It proposes that abnormal interactions among three cardinal networks—the self-representational default mode network, the behavioral relevance-encoding salience network and the goal-oriented central executive network—underlie brain disorders. Tinnitus commonly leads to negative cognitive, emotional, and autonomic responses, phenomenologically expressed as tinnitus-related suffering, processed by the medial pathway. This anatomically overlaps with the salience network, encoding the behavioral relevance of the sound stimulus. Chronic tinnitus can also become associated with the self-representing default mode network and becomes an intrinsic part of the self-percept. This is likely an energy-saving evolutionary adaptation, by detaching tinnitus from sympathetic energy-consuming activity. Eventually, this can lead to functional disability by interfering with the central executive network. In conclusion, these three pathways can be extended to a triple network model explaining all tinnitus-associated comorbidities. This model paves the way for the development of individualized treatment modalities.
Tinnitus can be defined as the conscious awareness of a tonal and/or noise sound in the milieu of no identifiable corresponding external acoustic source [1]. By contrast, tinnitus disorder is defined as tinnitus with tinnitus-associated suffering, which can consist of emotional distress, cognitive dysfunction and/or autonomic arousal (i.e., stress), leading to functional disability [1]. In other words, tinnitus can be equated to the perception of a phantom sound, and tinnitus disorder is tinnitus accompanied by associated suffering, with or without functional disability. It has been shown that chronic tinnitus (tinnitus with a duration of 3 months or more [1]) is not simply a temporal extension of tinnitus of recent onset but involves distinct mechanisms [2,3]. Tinnitus has a high prevalence, between 12% and 30% [4], increases with age, is more common in men than women, and is expressed more in the left than right ear [4].
The phantom sound is often associated with comorbidities, especially in the auditory domain, such as hearing loss (80% in the elderly) [5] and hyperacusis (7%–70%) [6] being the most common ones. However, tinnitus is also frequently associated with emotional, cognitive, and autonomic problems, thereby qualifying as tinnitus disorder. Indeed, stress is common in tinnitus patients (27%) [7], as are anxiety (26%) and depression (26%). Insomnia is present in 60%–73% of tinnitus patients [8,9]. Tinnitus is also associated with poorer performance across a variety of broad cognitive domains including executive function, cognitive processing speed, short-term memory, and general learning and memory retrieval [10]. This is mainly due to tinnitus-related distress [11]. The natural course of tinnitus is unfavorable. In 18% of patients tinnitus resolves spontaneously in 4 years, while in the other 82% of patients in whom tinnitus persists, it improves in 9% and worsens in 9% [12].
The mainstay of tinnitus treatment is cognitive behavioral therapy (CBT), for which there is meta-analytic evidence of its benefits [13]. Yet, CBT results only in a 10% improvement of the distress, without decreasing the loudness [13]. Furthermore, the meta-analysis could not find a benefit 6 months after CBT treatment [13], and it is highly uncertain whether CBT reduces anxiety, improves health-related quality of life, or reduces negatively biased interpretations of tinnitus [13]. To develop more efficacious tinnitus treatments, a better understanding of the pathophysiological mechanisms that generate and maintain chronic tinnitus is required. Better treatments should treat both the sound and the suffering. Pain and tinnitus share an analogous pathophysiology [14-25], clinical phenomenology [14,19-21,23], and neuromodulatory treatment approaches [19-21,23,26,27].
Chronic tinnitus has been anatomically and symptomatically dissociated into three separable but interacting ascending or descending pathways [16], analogous to what has been described for pain [16,28-33]. These consist of an ascending lateral “sound” pathway, an ascending non-specific medial “suffering” pathway, and a descending noise-canceling pathway [16,34,35]. The medial suffering pathway may overlap with the salience network, analogous to what has been shown for pain [29]. Whereas the lateral auditory and medial salience pathways can explain the loudness and emotional aspects of tinnitus respectively, no correlates have been proposed for the chronification and functional disability encountered in chronic tinnitus. We here propose to extend the current tinnitus networks to the triple cortical network model to fill this gap.
The triple network model is a network science-based approach to find a common framework for understanding cognitive and affective disorders [36]. It proposes that abnormal interactions within and between the three cardinal brain networks underlie neuropsychiatric disorders [36]. These three networks include the self-representational default mode network (i.e., a brain network that is active when a person is not focused on the outside world) [37,38], the behavioral relevance–encoding salience network (i.e., a brain network that selects which stimuli are salient and thus deserving of our attention) [39], and the goal-oriented frontoparietal central executive network (i.e., a brain network crucial for maintaining and processing information in working memory, problem-solving and decision-making) [39,40]. Normally, the central executive network and salience network demonstrate correlated activity, and both networks are anti-correlated to the default mode network [41]. The salience network drives the switch between the anti-correlated default mode network and the central executive network [42-44]. This is in keeping with the proposed functions of the three networks. When the salience network detects a behaviorally relevant external stimulus, it decreases the activity of the self-oriented and mind-wandering default mode network and activates the central executive network to address the salient external stimulus in a goal-oriented fashion. In many brain disorders, such as attention-deficit/hyperactivity disorder, anxiety, depression, bipolar, autism, obsessive-compulsive disorder, posttraumatic stress disorder, and schizophrenia, the functional connectivity (statistical relationships between a pair of brain regions that covary or correlate over time) within and between these three cardinal networks is aberrant [36,45,46].
We propose that in chronic tinnitus, analogous to what has been suggested for pain [47], the three known tinnitus pathways can be linked and extended to the triple network model, which would explain the chronification of tinnitus as well as propose neural correlates for the commonly associated cognitive dysfunction.
A stimulus produces an effect on the different sensory receptors, which is transmitted to the sensory cortex, inducing sensation [14]. Further processing of this sensory stimulation by other brain networks such as the default mode, salience network, and central executive network (i.e., the triple network) permits the sound stimulus to reach consciousness [48-53], and generates an internal representation of the outer and inner world, namely a percept [14]. Tinnitus perception can thus be defined as the act of interpreting and organizing a sound stimulus to produce a meaningful experience of the world and of oneself [14].
When a patient visits a health care provider and states that he or she has tinnitus, what the person is really conveying is, “I have a certain amount of tinnitus loudness associated with a certain amount of suffering during a certain amount of time.” These three aspects of the unified tinnitus percept can be traced to the three different pathways involved in tinnitus processing (Fig. 1) [16].
As mentioned, the two main tinnitus-activating pathways include the anatomically and functionally separable medial and lateral tinnitus pathways [16]. The medial ascending pathway involves the rostral to dorsal anterior cingulate cortex (rdACC) and anterior insular cortex and processes the affective motivational aspect of tinnitus [14,16,54-56]. The causality has been demonstrated by the fact that cingulotomies could abate negative affect and cognitive control [57,58], as researchers stated: “All the patients except two found the head noises less distressing after the operation but objectively the noises were unchanged” [57].
The lateral ascending pathway involving the auditory cortex encodes the discriminatory components of the tinnitus, such as loudness [59] and tinnitus localization [60]. The two activating tinnitus pathways are balanced by the noise-canceling inhibitory pathway, involving the pregenual anterior cingulate cortex (pgACC) [16,53]. The descending noise-canceling pathway determines the amount of time that tinnitus is consciously perceived [61,62], and therefore reflects the capacity of the brain to suppress acute or ongoing tinnitus [61].
As above-mentioned, tinnitus can be defined as the conscious awareness of a tonal and/or noise sound for which there is no identifiable corresponding external acoustic source, while tinnitus disorder is defined as tinnitus with suffering [1]. Suffering can be defined as an unpleasant experience associated with negative cognitive, emotional, and autonomic responses to a (tinnitus) stimulus [29]. About 80% of the people with tinnitus are not bothered by the phantom sound, but in 20% it is severely bothersome [63], qualifying as tinnitus disorder [1].
The sensation of a phantom sound can lead to suffering via the associated feeling of (emotional) unpleasantness and (cognitive) catastrophizing. Tinnitus catastrophizing is characterized by (1) a tendency to magnify the threat value of tinnitus, (2) feeling helpless in the context of tinnitus, and (3) a relative inability to inhibit tinnitus-related thoughts (rumination) [64]. Thus, tinnitus catastrophizing acts as an amplifier of unpleasantness and tinnitus loudness by deficient cognitive coping strategies [65]. The combination of the perceived unpleasantness and catastrophizing leads to suffering, which can express in different behaviors such as anger, fear, and frustration [64,65] (Fig. 1).
Tinnitus is often associated with stress [7,66-68]. Physiological stress can be defined as an unpleasant sensory, emotional, and subjective experience that is associated with potential damage of body tissue and bodily threat [69], especially when an environmental demand exceeds the natural regulatory capacity of an organism [70]. Stress results in an immediate adaptive temporary response of the autonomic nervous system and slower protracted stimulation of the hypothalamic-pituitary adrenal endocrine axis [71]. Whereas acute stress responses are adaptive and beneficial to survival by preparing a fight-or-flight response, chronic stress becomes maladaptive, leading to a host of problems including metabolic syndrome, obesity, cancer, mental health disorders, cardiovascular disease, and increased susceptibility to infections [72]. Chronic stress can result in depression [72,73], anxiety [73], and anger [74]—in other words, chronic suffering.
Tinnitus distress correlates with activity in the rdACC [55,56,75]. The increased cortisol level in stress results in unpleasantness via its functional modulation of the rdACC [76]. The neural substrates of physiological stress, as identified by a meta-analysis also involve the rdACC and anterior insula [69]. Unpleasantness (suffering) and sound intensity (loudness) can be modulated independently. This has been known since the 1950s, as following frontal lobotomies performed for tinnitus it was realized that “In fact, of the 19 patients who survived the operation, 11 felt that their head noises were just the same but bothered them less and 8 felt that they had improved” [57]. Similar observations were made by other neurosurgeons [58] and are equivalent to changes noted with electrode implants in the rdACC [54].
In summary, tinnitus consists of a sensory loudness component, encoded by the ascending lateral pathway, and a suffering component, encoded by the ascending medial pathway. Suffering involves a cognitive, emotional, and autonomic component, all encoded by parts of the medial pathway. The medial and lateral pathways are separable, and consequently, one may have tinnitus without suffering and suffering without tinnitus.
Network science is a research field studying complex networks such as computer, economic, biological, social, cognitive, and semantic networks, abstracting the networks to nodes (or vertices) and their connections (or edges). Network science is increasingly used to understand the involvement of resting-state network interactions in brain disorders [77-80], including tinnitus [81,82]. One of its findings is the involvement of the default mode network in tinnitus [83-91]. It can be hypothesized that in chronic tinnitus the default mode network, which controls self-representational processing may become pathologically connected to tinnitus-provoking networks [89]. The significance of this finding is tremendous, as this may be a neurobiological rationale why in chronic tinnitus the sound becomes embodied—that is, an integral part of the self, the new normal default state [92]—thereby making treatments more difficult [93]. Furthermore, not only can tinnitus become an integral part of the self, but when suffering becomes chronic, fear can turn into anxiety and sadness into depression, all common comorbidities in tinnitus.
A question can be raised, “why does this connectivity to the default mode occur?” An evolutionary explanation can be proposed that involves the free energy principle of brain functioning [94,95]. In sum, it posits that an energy-expensive organ, such as the brain, tries to conserve energy in whatever way it can [96,97]. It is of interest that the salience network and the sympathetic central control overlap in the brain [98], and the central component of the brain’s parasympathetic nervous network partially overlaps with the default mode network [99].
The sympathetic nervous system increases intrinsic energy consumption by 15%–35% [100,101]. In recent-onset pain, the daily energy consumption is increased by 60% [102], whereas in more chronic pain, the daily extra energy expenditure is only increased by 15% [103,104]. Similarly, fear increases energy expenditure by 22% [105], whereas chronic anxiety only increases energy expenditure by 6% [106]. By rewiring to connect the tinnitus pathways to the default mode network, which broadly overlaps with the parasympathetic central network, energy expenditure can therefore be saved. Data on energy expenditure in acute tinnitus versus chronic tinnitus have not been published yet, but might hopefully be performed, as to verify whether this concept holds for tinnitus as well.
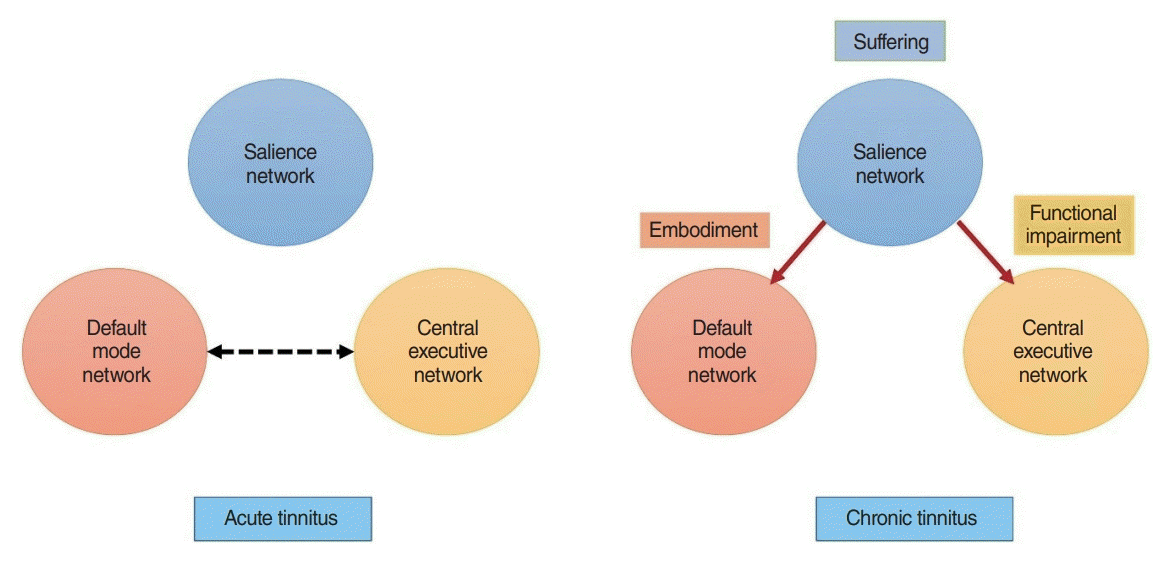
Chronic tinnitus can lead to a lower quality of life and the development of tinnitus-related disability, especially cognitive disability [10,11,107,108]. Tinnitus loudness, tinnitus distress, and tinnitus duration correlate positively with different cognitive measures (e.g., the trail-making test, Montreal cognitive assessment, Mini-Mental State Examination) [109]. Based on network science principles, each aspect of tinnitus could be the result of connectivity changes between the lateral pathway (i.e., the auditory network), and another resting-state network, such as the salience network (suffering), the default mode network (embodiment), the central executive network (cognitive disability), and motor network (physical disability). Changes in the auditory [110,111], salience [112-116], default mode [83-91], and central executive networks [87,113] have been shown in patients with tinnitus. In a healthy state, the salience network, which overlaps with the ascending medial pathway, and the stress network are anti-correlated with the default mode network [41]. In chronic tinnitus, this anti-correlation is lost, as suggested by increased functional connectivity between the rdACC and precuneus [91]. Furthermore, interference in the goal-oriented central executive network may lead to functional impairment in subjects with tinnitus (Fig. 2).
Tinnitus is processed by three distinguishable but interacting networks, each encoding different tinnitus characteristics. The ascending lateral pathway, with the auditory cortex as the main hub, is responsible predominantly for loudness. The ascending medial pathway, with the rdACC and insula as the main hubs, are involved in the suffering component, and the descending noise-canceling pathway, with the pgACC as the main hub, is related to the percentage of the time that the tinnitus is present. When the tinnitus sensation pathways become correlated to the default mode instead of anti-correlated, tinnitus becomes part of the self-percept (i.e., part of who one is, the new normal). This can subsequently lead to functional disability by interfering with the goal-oriented central executive network. Therefore, the three tinnitus pathways previously described need to be extended to incorporate the triple network to explain the full clinical picture of chronic tinnitus.
▪ The triple network model is a novel unifying framework common to neuropsychiatric disorders.
▪ The salience network encodes the behavioral relevance of tinnitus.
▪ The default mode network makes tinnitus an intrinsic part of the self-percept.
▪ Tinnitus can lead to functional disability by interfering with the central executive network.
Notes
AUTHOR CONTRIBUTIONS
Conceptualization: DDR, SV. Data curation: DDR. Formal analysis: DDR. Investigation: DDR, SV, DA. Methodology: DDR, SV, DA. Project administration: DDR, SV, DA. Resources: DDR, SV, DA. Software: JJS. Supervision: DDR. Validation: JJS. Visualization: JJS. Writing–original draft: DDR, SV, DA. Writing–review & editing: JJS.
ACKNOWLEDGMENTS
This work was supported by grants from the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (grant No. NRF-2022R1A2B5B02002139 to JJS), from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare (grant No. HI21C1574 to JJS), and from Seoul National University Bundang Hospital (grant No. 14-2021-0032 to JJS).
REFERENCES
1. De Ridder D, Schlee W, Vanneste S, Londero A, Weisz N, Kleinjung T, et al. Tinnitus and tinnitus disorder: theoretical and operational definitions (an international multidisciplinary proposal). Prog Brain Res. 2021; 260:1–25.
2. Vanneste S, van de Heyning P, De Ridder D. The neural network of phantom sound changes over time: a comparison between recentonset and chronic tinnitus patients. Eur J Neurosci. 2011; Sep. 34(5):718–31.
3. Wallhausser-Franke E, D’Amelio R, Glauner A, Delb W, Servais JJ, Hormann K, et al. Transition from acute to chronic tinnitus: predictors for the development of chronic distressing tinnitus. Front Neurol. 2017; Nov. 8:605.
4. McCormack A, Edmondson-Jones M, Somerset S, Hall D. A systematic review of the reporting of tinnitus prevalence and severity. Hear Res. 2016; Jul. 337:70–9.
5. Gibrin PC, Melo JJ, Marchiori LL. Prevalence of tinnitus complaints and probable association with hearing loss, diabetes mellitus and hypertension in elderly. Codas. 2013; 25(2):176–80.
6. Baguley DM, Hoare DJ. Hyperacusis: major research questions. HNO. 2018; May. 66(5):358–63.
7. Park E, Kim H, Choi IH, Han HM, Han K, Jung HH, et al. Psychiatric distress as a common risk factor for tinnitus and joint pain: a national population-based survey. Clin Exp Otorhinolaryngol. 2020; Aug. 13(3):234–40.
8. Cronlein T, Langguth B, Pregler M, Kreuzer PM, Wetter TC, Schecklmann M. Insomnia in patients with chronic tinnitus: cognitive and emotional distress as moderator variables. J Psychosom Res. 2016; Apr. 83:65–8.
9. Asnis GM, Henderson M, Sylvester C, Thomas M, Kiran M, Richard De La G. Insomnia in tinnitus patients: a prospective study finding a significant relationship. Int Tinnitus J. 2021; Jan. 24(2):65–9.
10. Clarke NA, Henshaw H, Akeroyd MA, Adams B, Hoare DJ. Associations between subjective tinnitus and cognitive performance: systematic review and meta-analyses. Trends Hear. 2020; Jan-Dec. 24:2331216520918416.
11. Neff P, Simoes J, Psatha S, Nyamaa A, Boecking B, Rausch L, et al. The impact of tinnitus distress on cognition. Sci Rep. 2021; Jan. 11(1):2243.
12. Dawes P, Newall J, Stockdale D, Baguley DM. Natural history of tinnitus in adults: a cross-sectional and longitudinal analysis. BMJ Open. 2020; Dec. 10(12):e041290.
13. Fuller T, Cima R, Langguth B, Mazurek B, Vlaeyen JW, Hoare DJ. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2020; Jan. 1. (1):CD012614.
14. De Ridder D, Elgoyhen AB, Romo R, Langguth B. Phantom percepts: tinnitus and pain as persisting aversive memory networks. Proc Natl Acad Sci U S A. 2011; May. 108(20):8075–80.
15. De Ridder D, Van de Heyning P. The Darwinian plasticity hypothesis for tinnitus and pain. Prog Brain Res. 2007; 166:55–60.
16. De Ridder D, Vanneste S. The Bayesian brain in imbalance: medial, lateral and descending pathways in tinnitus and pain: a perspective. Prog Brain Res. 2021; 262:309–34.
17. Jeanmonod D, Magnin M, Morel A. Low-threshold calcium spike bursts in the human thalamus: common physiopathology for sensory, motor and limbic positive symptoms. Brain. 1996; Apr. 119(Pt 2):363–75.
18. Llinas RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci U S A. 1999; Dec. 96(26):15222–7.
19. Moller AR. Similarities between severe tinnitus and chronic pain. J Am Acad Audiol. 2000; Mar. 11(3):115–24.
20. Moller AR. Similarities between chronic pain and tinnitus. Am J Otol. 1997; Sep. 18(5):577–85.
21. Moller AR. Tinnitus and pain. Prog Brain Res. 2007; 166:47–53.
22. Rauschecker JP, May ES, Maudoux A, Ploner M. Frontostriatal gating of tinnitus and chronic pain. Trends Cogn Sci. 2015; Oct. 19(10):567–78.
23. Tonndorf J. The analogy between tinnitus and pain: a suggestion for a physiological basis of chronic tinnitus. Hear Res. 1987; 28(2-3):271–5.
24. Vanneste S, Song JJ, De Ridder D. Thalamocortical dysrhythmia detected by machine learning. Nat Commun. 2018; Mar. 9(1):1103.
25. Vanneste S, To WT, De Ridder D. Tinnitus and neuropathic pain share a common neural substrate in the form of specific brain connectivity and microstate profiles. Prog Neuropsychopharmacol Biol Psychiatry. 2019; Jan. 88:388–400.
26. De Ridder D, De Mulder G, Menovsky T, Sunaert S, Kovacs S. Electrical stimulation of auditory and somatosensory cortices for treatment of tinnitus and pain. Prog Brain Res. 2007; 166:377–88.
27. De Ridder D, Moller AR. Similarities between treatments of tinnitus and central pain. In : Moller AR, Langguth B, De Ridder D, Kleinjung T, editors. Textbook of tinnitus. New York (NY): Springer;2011. p. 753–62.
28. Al-Chalabi M, Reddy V, Gupta S. Neuroanatomy, spinothalamic tract [Internet]. Treasure Island (FL): StatPearls Publishing;2022 [cited 2022 Jul 1]. Available from: https://pubmed.ncbi.nlm.nih.gov/29939601/.
29. De Ridder D, Adhia D, Vanneste S. The anatomy of pain and suffering in the brain and its clinical implications. Neurosci Biobehav Rev. 2021; Nov. 130:125–46.
30. De Ridder D, Vanneste S. Burst and tonic spinal cord stimulation: different and common brain mechanisms. Neuromodulation. 2016; Jan. 19(1):47–59.
31. Frot M, Mauguiere F, Magnin M, Garcia-Larrea L. Parallel processing of nociceptive A-delta inputs in SII and midcingulate cortex in humans. J Neurosci. 2008; Jan. 28(4):944–52.
32. Vanneste S, De Ridder D. Chronic pain as a brain imbalance between pain input and pain suppression. Brain Commun. 2021. Feb. 3. (1):fcab014.
33. Yearwood T, De Ridder D, Yoo HB, Falowski S, Venkatesan L, Ting To W, et al. Comparison of neural activity in chronic pain patients during tonic and burst spinal cord stimulation using fluorodeoxyglucose positron emission tomography. Neuromodulation. 2020; Jan. 23(1):56–63.
34. Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013; Jul. 14(7):502–11.
35. Rainville P, Carrier B, Hofbauer RK, Bushnell CM, Duncan GH. Dissociation of sensory and affective dimensions of pain using hypnotic modulation. Pain. 1999; Aug. 82(2):159–71.
36. Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011; Oct. 15(10):483–506.
37. Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003; Jan. 100(1):253–8.
38. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008; Mar. 1124:1–38.
39. Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007; Feb. 27(9):2349–56.
40. Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008; Dec. 100(6):3328–42.
41. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005; Jul. 102(27):9673–8.
42. Goulden N, Khusnulina A, Davis NJ, Bracewell RM, Bokde AL, McNulty JP, et al. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. Neuroimage. 2014; Oct. 99:180–90.
43. Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010; Jun. 214(5-6):655–67.
44. Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008; Aug. 105(34):12569–74.
45. Sha Z, Wager TD, Mechelli A, He Y. Common dysfunction of large-scale neurocognitive networks across psychiatric disorders. Biol Psychiatry. 2019; Mar. 85(5):379–88.
46. Sha Z, Xia M, Lin Q, Cao M, Tang Y, Xu K, et al. Meta-connectomic analysis reveals commonly disrupted functional architectures in network modules and connectors across brain disorders. Cereb Cortex. 2018; Dec. 28(12):4179–94.
47. De Ridder D, Vanneste S, Smith M, Adhia D. Pain and the triple network model. Front Neurol. 2022; Mar. 13:757241.
48. Boly M, Faymonville ME, Peigneux P, Lambermont B, Damas F, Luxen A, et al. Cerebral processing of auditory and noxious stimuli in severely brain injured patients: differences between VS and MCS. Neuropsychol Rehabil. 2005; Jul-Sep. 15(3-4):283–9.
49. Boly M, Faymonville ME, Peigneux P, Lambermont B, Damas P, Del Fiore G, et al. Auditory processing in severely brain injured patients: differences between the minimally conscious state and the persistent vegetative state. Arch Neurol. 2004; Feb. 61(2):233–8.
50. Boly M, Garrido MI, Gosseries O, Bruno MA, Boveroux P, Schnakers C, et al. Preserved feedforward but impaired top-down processes in the vegetative state. Science. 2011; May. 332(6031):858–62.
51. Laureys S, Faymonville ME, Degueldre C, Fiore GD, Damas P, Lambermont B, et al. Auditory processing in the vegetative state. Brain. 2000; Aug. 123(Pt 8):1589–601.
52. Laureys S, Perrin F, Faymonville ME, Schnakers C, Boly M, Bartsch V, et al. Cerebral processing in the minimally conscious state. Neurology. 2004; Sep. 63(5):916–8.
53. De Ridder D, Vanneste S, Weisz N, Londero A, Schlee W, Elgoyhen AB, et al. An integrative model of auditory phantom perception: tinnitus as a unified percept of interacting separable subnetworks. Neurosci Biobehav Rev. 2014; Jul. 44:16–32.
54. De Ridder D, Joos K, Vanneste S. Anterior cingulate implants for tinnitus: report of 2 cases. J Neurosurg. 2016; Apr. 124(4):893–901.
55. Vanneste S, Plazier M, der Loo Ev, de Heyning PV, Congedo M, De Ridder D. The neural correlates of tinnitus-related distress. Neuroimage. 2010; Aug. 52(2):470–80.
56. De Ridder D, Vanneste S, Congedo M. The distressed brain: a group blind source separation analysis on tinnitus. PLoS One. 2011; 6(10):e24273.
57. Beard AW. Results of leucotomy operations for tinnitus. J Psychosom Res. 1965; Sep. 9(1):29–32.
58. Elithorn A. Prefrontal leucotomy in the treatment of tinnitus. Proc R Soc Med. 1953; Oct. 46(10):832–3.
59. van der Loo E, Gais S, Congedo M, Vanneste S, Plazier M, Menovsky T, et al. Tinnitus intensity dependent gamma oscillations of the contralateral auditory cortex. PLoS One. 2009; Oct. 4(10):e7396.
60. Smits M, Kovacs S, de Ridder D, Peeters RR, van Hecke P, Sunaert S. Lateralization of functional magnetic resonance imaging (fMRI) activation in the auditory pathway of patients with lateralized tinnitus. Neuroradiology. 2007; Aug. 49(8):669–79.
61. Song JJ, Vanneste S, De Ridder D. Dysfunctional noise cancelling of the rostral anterior cingulate cortex in tinnitus patients. PLoS One. 2015; Apr. 10(4):e0123538.
62. Seydell-Greenwald A, Leaver AM, Turesky TK, Morgan S, Kim HJ, Rauschecker JP. Functional MRI evidence for a role of ventral prefrontal cortex in tinnitus. Brain Res. 2012; Nov. 1485:22–39.
63. Axelsson A, Ringdahl A. Tinnitus: a study of its prevalence and characteristics. Br J Audiol. 1989; Feb. 23(1):53–62.
64. Cima RF, Crombez G, Vlaeyen JW. Catastrophizing and fear of tinnitus predict quality of life in patients with chronic tinnitus. Ear Hear. 2011; Sep-Oct. 32(5):634–41.
65. Weise C, Hesser H, Andersson G, Nyenhuis N, Zastrutzki S, Kroner-Herwig B, et al. The role of catastrophizing in recent onset tinnitus: its nature and association with tinnitus distress and medical utilization. Int J Audiol. 2013; Mar. 52(3):177–88.
66. Hebert S, Lupien SJ. The sound of stress: blunted cortisol reactivity to psychosocial stress in tinnitus sufferers. Neurosci Lett. 2007; Jan. 411(2):138–42.
67. Hebert S, Lupien SJ. Salivary cortisol levels, subjective stress, and tinnitus intensity in tinnitus sufferers during noise exposure in the laboratory. Int J Hyg Environ Health. 2009; Jan. 212(1):37–44.
68. Hinton DE, Chhean D, Pich V, Hofmann SG, Barlow DH. Tinnitus among Cambodian refugees: relationship to PTSD severity. J Trauma Stress. 2006; Aug. 19(4):541–6.
69. Kogler L, Muller VI, Chang A, Eickhoff SB, Fox PT, Gur RC, et al. Psychosocial versus physiological stress: meta-analyses on deactivations and activations of the neural correlates of stress reactions. Neuroimage. 2015; Oct. 119:235–51.
70. Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flugge G, Korte SM, et al. Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev. 2011; Apr. 35(5):1291–301.
71. Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009; Jun. 10(6):397–409.
72. Russell G, Lightman S. The human stress response. Nat Rev Endocrinol. 2019; Sep. 15(9):525–34.
73. Staufenbiel SM, Penninx BW, Spijker AT, Elzinga BM, van Rossum EF. Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology. 2013; Aug. 38(8):1220–35.
74. Gilam G, Lin T, Fruchter E, Hendler T. Neural indicators of interpersonal anger as cause and consequence of combat training stress symptoms. Psychol Med. 2017; Jul. 47(9):1561–72.
75. Joos K, Vanneste S, De Ridder D. Disentangling depression and distress networks in the tinnitus brain. PLoS One. 2012; 7(7):e40544.
76. Vachon-Presseau E, Martel MO, Roy M, Caron E, Albouy G, Marin MF, et al. Acute stress contributes to individual differences in pain and pain-related brain activity in healthy and chronic pain patients. J Neurosci. 2013; Apr. 33(16):6826–33.
77. Telesford QK, Simpson SL, Burdette JH, Hayasaka S, Laurienti PJ. The brain as a complex system: using network science as a tool for understanding the brain. Brain Connect. 2011; Oct. 1(4):295–308.
78. Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009; Mar. 10(3):186–98.
79. Sporns O, Chialvo DR, Kaiser M, Hilgetag CC. Organization, development and function of complex brain networks. Trends Cogn Sci. 2004; Sep. 8(9):418–25.
80. Suarez LE, Markello RD, Betzel RF, Misic B. Linking structure and function in macroscale brain networks. Trends Cogn Sci. 2020; Apr. 24(4):302–15.
81. Mohan A, De Ridder D, Vanneste S. Graph theoretical analysis of brain connectivity in phantom sound perception. Sci Rep. 2016; Feb. 6:19683.
82. Elgoyhen AB, Langguth B, Vanneste S, De Ridder D. Tinnitus: network pathophysiology-network pharmacology. Front Syst Neurosci. 2012; Jan. 6:1.
83. Schmidt SA, Akrofi K, Carpenter-Thompson JR, Husain FT. Default mode, dorsal attention and auditory resting state networks exhibit differential functional connectivity in tinnitus and hearing loss. PLoS One. 2013; Oct. 8(10):e76488.
84. Song JJ, Park J, Koo JW, Lee SY, Vanneste S, De Ridder D, et al. The balance between Bayesian inference and default mode determines the generation of tinnitus from decreased auditory input: a volume entropy-based study. Hum Brain Mapp. 2021; Aug. 42(12):4059–73.
85. Eggermont JJ. Separate auditory pathways for the induction and maintenance of tinnitus and hyperacusis. Prog Brain Res. 2021; 260:101–27.
86. Hu J, Cui J, Xu JJ, Yin X, Wu Y, Qi J. The neural mechanisms of tinnitus: a perspective from functional magnetic resonance imaging. Front Neurosci. 2021; Feb. 15:621145.
87. Zhou GP, Shi XY, Wei HL, Qu LJ, Yu YS, Zhou QQ, et al. Disrupted intraregional brain activity and functional connectivity in unilateral acute tinnitus patients with hearing loss. Front Neurosci. 2019; Sep. 13:1010.
88. Chen YC, Zhang H, Kong Y, Lv H, Cai Y, Chen H, et al. Alterations of the default mode network and cognitive impairment in patients with unilateral chronic tinnitus. Quant Imaging Med Surg. 2018; Nov. 8(10):1020–9.
89. Schmidt SA, Carpenter-Thompson J, Husain FT. Connectivity of precuneus to the default mode and dorsal attention networks: a possible invariant marker of long-term tinnitus. Neuroimage Clin. 2017; Jul. 16:196–204.
90. Lanting C, WozAniak A, van Dijk P, Langers DR. Tinnitus- and task-related differences in resting-state networks. Adv Exp Med Biol. 2016; Apr. 894:175–87.
91. Chen YC, Chen H, Bo F, Xu JJ, Deng Y, Lv H, et al. Tinnitus distress is associated with enhanced resting-state functional connectivity within the default mode network. Neuropsychiatr Dis Treat. 2018; Aug. 14:1919–27.
92. Lee SY, Choi BY, Koo JW, De Ridder D, Song JJ. Cortical oscillatory signatures reveal the prerequisites for tinnitus perception: a comparison of subjects with sudden sensorineural hearing loss with and without tinnitus. Front Neurosci. 2020; Nov. 14:596647.
93. Han JJ, Ridder D, Vanneste S, Chen YC, Koo JW, Song JJ. Pre-treatment ongoing cortical oscillatory activity predicts improvement of tinnitus after partial peripheral reafferentation with hearing aids. Front Neurosci. 2020; May. 14:410.
94. Friston K. The free-energy principle: a unified brain theory. Nat Rev Neurosci. 2010; Feb. 11(2):127–38.
95. Friston K. The free-energy principle: a rough guide to the brain. Trends Cogn Sci. 2009; Jul. 13(7):293–301.
96. Hitze B, Hubold C, van Dyken R, Schlichting K, Lehnert H, Entringer S, et al. How the selfish brain organizes its supply and demand. Front Neuroenergetics. 2010; Jun. 2:7.
97. Peters A, Schweiger U, Pellerin L, Hubold C, Oltmanns KM, Conrad M, et al. The selfish brain: competition for energy resources. Neurosci Biobehav Rev. 2004; Apr. 28(2):143–80.
98. Taylor KS, Kucyi A, Millar PJ, Murai H, Kimmerly DS, Morris BL, et al. Association between resting-state brain functional connectivity and muscle sympathetic burst incidence. J Neurophysiol. 2016; Feb. 115(2):662–73.
99. Babo-Rebelo M, Richter CG, Tallon-Baudry C. Neural responses to heartbeats in the default network encode the self in spontaneous thoughts. J Neurosci. 2016; Jul. 36(30):7829–40.
100. Sjostrom L, Schutz Y, Gudinchet F, Hegnell L, Pittet PG, Jequier E. Epinephrine sensitivity with respect to metabolic rate and other variables in women. Am J Physiol. 1983; Nov. 245(5 Pt 1):E431–42.
101. Fellows IW, Bennett T, MacDonald IA. The effect of adrenaline upon cardiovascular and metabolic functions in man. Clin Sci (Lond). 1985; Aug. 69(2):215–22.
102. Holland-Fischer P, Greisen J, Grofte T, Jensen TS, Hansen PO, Vilstrup H. Increased energy expenditure and glucose oxidation during acute nontraumatic skin pain in humans. Eur J Anaesthesiol. 2009; Apr. 26(4):311–7.
103. Xu Z, Li Y, Wang J, Li J. Effect of postoperative analgesia on energy metabolism and role of cyclooxygenase-2 inhibitors for postoperative pain management after abdominal surgery in adults. Clin J Pain. 2013; Jul. 29(7):570–6.
104. Straub RH. The brain and immune system prompt energy shortage in chronic inflammation and ageing. Nat Rev Rheumatol. 2017; Dec. 13(12):743–51.
105. Whitehorn JC, Lundholm H, Gardner GE. The metabolic rate in emotional moods induced by suggestion in hypnosis. Am J Psychiatry. 1930; Jan. 86(4):661–6.
106. Schmidt WD, O’Connor PJ, Cochrane JB, Cantwell M. Resting metabolic rate is influenced by anxiety in college men. J Appl Physiol (1985). 1996; Feb. 80(2):638–42.
107. Mohamad N, Hoare DJ, Hall DA. The consequences of tinnitus and tinnitus severity on cognition: a review of the behavioural evidence. Hear Res. 2016; Feb. 332:199–209.
108. Tegg-Quinn S, Bennett RJ, Eikelboom RH, Baguley DM. The impact of tinnitus upon cognition in adults: a systematic review. Int J Audiol. 2016; Oct. 55(10):533–40.
109. Vanneste S, Faber M, Langguth B, De Ridder D. The neural correlates of cognitive dysfunction in phantom sounds. Brain Res. 2016; Jul. 1642:170–9.
110. Maudoux A, Lefebvre P, Cabay JE, Demertzi A, Vanhaudenhuyse A, Laureys S, et al. Connectivity graph analysis of the auditory resting state network in tinnitus. Brain Res. 2012; Nov. 1485:10–21.
111. Maudoux A, Lefebvre P, Cabay JE, Demertzi A, Vanhaudenhuyse A, Laureys S, et al. Auditory resting-state network connectivity in tinnitus: a functional MRI study. PLoS One. 2012; May. 7(5):e36222.
112. Shahsavarani S, Schmidt SA, Khan RA, Tai Y, Husain FT. Salience, emotion, and attention: the neural networks underlying tinnitus distress revealed using music and rest. Brain Res. 2021; Mar. 1755:147277.
113. Kandeepan S, Maudoux A, Ribeiro de Paula D, Zheng JY, Cabay JE, Gomez F, et al. Tinnitus distress: a paradoxical attention to the sound. J Neurol. 2019; Sep. 266(9):2197–207.
114. Xu XM, Jiao Y, Tang TY, Lu CQ, Zhang J, Salvi R, et al. Altered spatial and temporal brain connectivity in the salience network of sensorineural hearing loss and tinnitus. Front Neurosci. 2019; Mar. 13:246.
115. Amaral AA, Langers DR. Tinnitus-related abnormalities in visual and salience networks during a one-back task with distractors. Hear Res. 2015; Aug. 326:15–29.
116. De Ridder D, Vanneste S, Freeman W. The Bayesian brain: phantom percepts resolve sensory uncertainty. Neurosci Biobehav Rev. 2014; Jul. 44:4–15.
Fig. 1.
The anatomical pathways associated with three different aspects of tinnitus (loudness, suffering, and presence). A sound stimulus leads to a cognitive, emotional, and autonomic response, which is phenomenologically expressed as catastrophizing, attention paid to the tinnitus, unpleasantness, fear, anger or frustration with tinnitus, and arousal/distress. These cognitive, emotional, and autonomic symptoms are all phenomenological expressions of altered activity in the medial pathway.
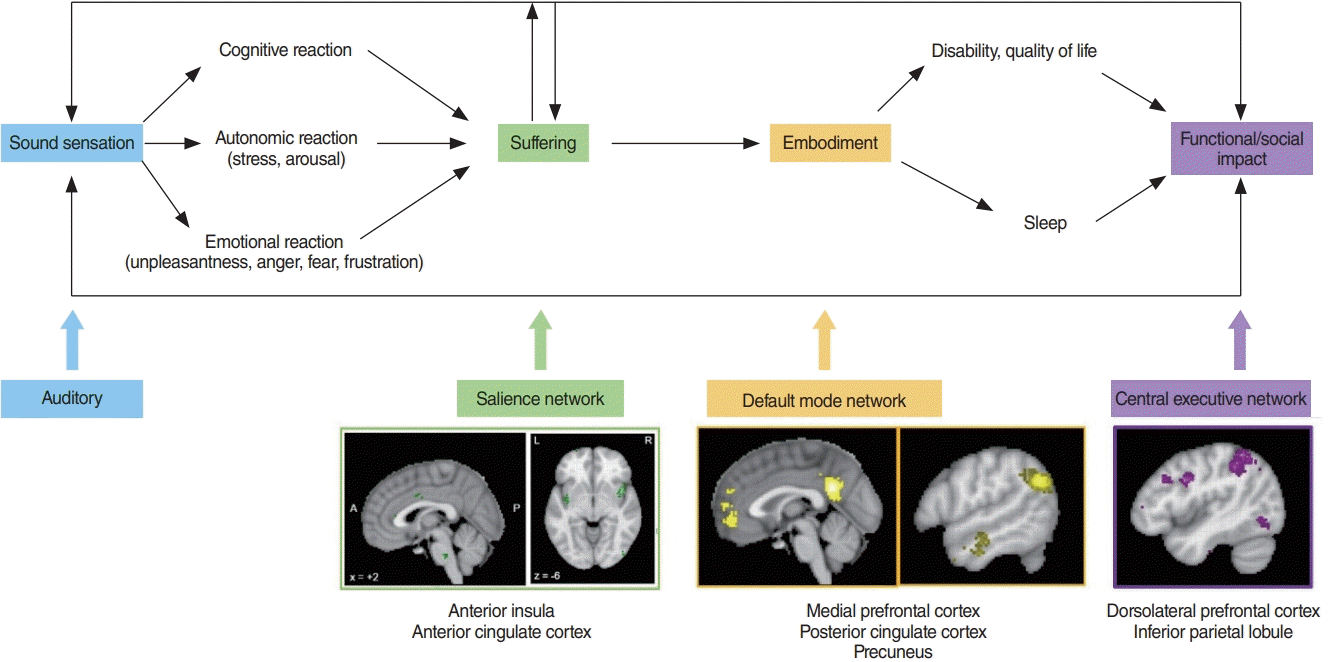
Fig. 2.
In subjects with acute tinnitus, the default mode network and the central executive network are anti-correlated. When tinnitus becomes chronic, the anticorrelation between the two networks disappear and the triple network including the salience network causes suffering, embodiment, and functional impairment because of tinnitus.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download