Abstract
Herpes zoster ophthalmicus (HZO) occurs due to reactivation of dormant varicella zoster virus infection in the ophthalmic division of the trigeminal nerve. Hutchinson’s sign, a herpetic skin lesion in the nasal tip, is a predictor of ocular complications, as the nasal tip area and ocular structure are innervated by the same nasociliary nerve. Patients who present with Hutchinson’s sign should be referred to an ophthalmologist due to possibility of ocular complications. Here, we present a case of a 44-year-old man with HZO that was confused with delayed rhinoplasty complication. The patient presented with nasal tip skin lesions 17 years after undergoing augmentation rhinoplasty. A graft-related infection was suspected due to operation history and skin lesions. However, surgical exploration disclosed no infection or inflammation, and serological tests revealed positive varicella zoster virus immunoglobulin M, and immunoglobulin G, antibodies. Based on these findings, the patient was diagnosed with HZO. Further, the patient received antiviral treatment with famciclovir. The lesions gradually improved with conservative treatment and became almost unrecognizable. Therefore, HZO should be considered when there is an unexplained skin lesion at the nasal tip in patients with history of rhinoplasty.
Herpes zoster (HZ) is a frequently presented disorder at primary care centers. It is caused by varicella-zoster virus (VZV, human herpes virus 3) that leads to varicella (chickenpox) as a primary infection [1]. Herpes zoster ophthalmicus (HZO) occurs due to reactivation of the dormant VZV in the trigeminal ganglia involving the ophthalmic branch [2]. Studies suggest that 50%–70% of patients with HZO experience direct ocular complication. It can be manifested as keratitis, episcleritis, iritis, conjunctivitis, and uveitis. However, HZO may manifest only as pain and rash, characteristic of HZ, without any involvement of the ocular structures [3,4].
The presence of herpetic skin lesions around the tip and side of the nose is termed as the Hutchinson’s sign. The presence of Hutchinson’s sign indicates involvement of the nasociliary branch that innervates the tip of the nose and cornea, as well as other ocular structures. Therefore, due to a high risk of ocular complications, Hutchinson’s sign requires urgent consultation with an ophthalmologist [3].
Here, we report a case with HZO confused with rhinoplasty related complication.
A 44-year-old man presented to the rhinoplasty clinic with a 7-day history of pain and skin lesions on the nose. The patient complained that the discomfort began from the right eye to nasal tip, along the side of the nose. The patient had no malaise or general fatigue. An initial physical examination indicated redness only at the nasal tip without swelling in the nasal dorsum. Seventeen years ago, the patient had undergone augmentation rhinoplasty with alloplastic graft material (Gore-Tex, expanded polytetrafluoroethylene [ePTFE]) at another tertiary hospital. We suspected a delayed post graft material-related infection, and administered empirical oral antibiotics. However, the skin lesion noticeably worsened after five days of administering the antibiotics. The nasal tip lesion became more necrotic, new skin lesions appeared on the nasal dorsum, and there were papules with punched ulcers (Fig. 1). Laboratory examination showed 4,930 white blood cells per mm3, and the other results were within normal limit.
The wound was immediately investigated with external rhinoplasty approach. Under general anesthesia, the V incision was made on the columella avoiding the necrotic lesion of the wound. The dissection was done over the upper and low lateral cartilage to explore the nasal tip and dorsum. There was no significant inflammation in the subcutaneous tissue of the nasal tip and nasal dorsum indicative of an infection at the rhinoplasty site (Figs. 2–4). Therefore, we suspected HZO, and orally administered 750 mg famciclovir antiviral treatment.
Following serologic tests revealed positive results for varicella zoster virus immunoglobulin M (IgM) antibody (1.35; reference ranges: negative, <0.9; borderline, 0.9–1.1; positive, >1.1) and immunoglobulin G (IgG) antibody (26.80; reference ranges: negative, <0.9; borderline, 0.9–1.1; positive, >1.1). However, there was no specific abnormal finding in ophthalmologic consultation.
Eight months after the surgery, the skin redness and crust at the nasal tip had resolved, and the wound had become almost unrecognizable (Fig. 5).
HZO occurs due to reactivation of the inactive VZV in the trigeminal ganglia involving the ophthalmic nerve [2]. It contributes to approximately 10%–20% of HZ cases [4], with the frontal branch being the most commonly involved. Approximately 50%–72% of cases with HZO may experience direct ocular involvement. HZO presents with variable appearances, including swelling, erythema, discharge, and tenderness. Moreover, HZO may manifest only as pain and rash characteristic of HZ without any involvement of the ocular structures [5].
The Hutchinson’s sign—the herpetic appearance of skin lesions at the tip and side of the nose—represents HZ infection of the dermatome of the nasociliary nerve. It has been used as a prognostic factor for subsequent ocular inflammation in patients with HZO [6]. Additionally, due to a high risk of ocular complication, the Hutchinson’s sign requires urgent consultation of an ophthalmologist [3]. Without antiviral therapy, approximately 50% of the patients with HZO may show ocular involvement. In severe cases, patients may also develop chronic blindness as a sequelae of HZO [7].
HZO is uncommon at rhinoplasty clinics, and on the other side, alloplastic graft-related infection is more common relatively. The mean percentage of infection after dorsum augmentation with alloplastic material was studied as 2.75% (95% CI, 1.61% to 4.17%) in the meta-analysis study [8]. In case of any sign of infection at the nasal tip area along with rhinoplasty history and no response to empirical antibiotic treatment, clinicians should perform surgical exploration to rule out any graft-related complication. A healthy operation bed should help suspect HZO. Moreover, serological tests for VZV (IgM and IgG; and polymerase chain reaction test for VZV) may aid in confirming HZO. In the present case, IgM and IgG for VZV were positive in the serological tests. Previous studies suggest a 10%–70% VZV IgM antibody positivity rate in cases with HZ depending on the method, period, and clinical symptoms of patients [6]. Also, the multiple herpetic skin lesions of nasal tip & dorsum can be helpful to diagnosis HZO, either. After diagnosis of HZO, antiviral therapy should be administered immediately [7].
There are several options for treating HZO, including oral acyclovir, valacyclovir, and famciclovir. Valacyclovir (1 g ter in die [TID]) and famciclovir (500 mg TID) are comparable in terms of effectiveness with acyclovir (800 mg 5 times a day) [9] both in the treatment of HZ and in reduction of complications. Intravenous acyclovir is recommended in immunosuppressed hosts, especially to prevent dissemination of disease, including herpes encephalitis [10]. The administration of oral corticosteroids with antiviral agents is recommended to reduce the duration of pain in the acute stage of HZO [11]. However, ophthalmic steroids should be administered cautiously, typically in direct consultation of an ophthalmologist as they can cause serious corneal complications [12]. A vaccination for VZV may help in preventing HZ or HZO, especially in immunocompromised individuals and adults aged >50 years [7].
The HZO presents with various complications, beginning with the skin and anterior segment of the ocular structure, and possibly involving the retina, optic nerve, and central nervous system [5]. Specifically, these complications include conjunctivitis, episcleritis, scleritis, uveitis, interstitial keratitis, secondary glaucoma, chronic corneal edema, acute retinal necrosis, optic neuropathy, meningoencephalitis, encephalitis, and stroke [13]. Additionally, post-herpetic neuralgia could be a significant complication of HZO that reduces patients’ overall quality of life [14]. Therefore, to prevent these complications, an early diagnosis of HZO is important [15].
In conclusion, HZO should be considered in the presence of an unexplained skin lesion at the nasal tip in patients with history of rhinoplasty.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
REFERENCES
1. Insinga RP, Itzler RF, Pellissier JM, Saddier P, Nikas AA. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med. 2005; 20(8):748–53.
2. Li A, Tandon A, Dinkin M, Oliveira C. The use of systemic steroids in the treatment of herpes zoster ophthalmicus-related ophthalmoplegia: case report and case meta-analysis. Am J Ophthalmol. 2021; (223):241–5.
3. Frary J, Petersen PT, Pareek M. Hutchinson’s sign of ophthalmic zoster. Clin Case Rep. 2020; 8(1):219–20.
4. Ragozzino MW, Melton LJ 3rd, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore). 1982; 61(5):310–6.
5. Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology. 2008; 115(2 Suppl):S3–12.
6. Gershon AA, Steinberg SP, Borkowsky W, Lennette D, Lennette E. IgM to varicella-zoster virus: demonstration in patients with and without clinical zoster. Pediatr Infect Dis. 1982; 1(3):164–7.
7. Davis AR, Sheppard J. Herpes zoster ophthalmicus review and prevention. Eye Contact Lens. 2019; 45(5):286–91.
8. Keyhan SO, Ramezanzade S, Yazdi RG, Valipour MA, Fallahi HR, Shakiba M, et al. Prevalence of complications associated with polymer-based alloplastic materials in nasal dorsal augmentation: a systematic review and meta-analysis. Maxillofac Plast Reconstr Surg. 2022; 44(1):17.
9. Marsh RJ, Cooper M. Oral acyclovir in acute herpes zoster. Br Med J (Clin Res Ed). 1987; 294(6573):704.
10. Balfour HH Jr. Varicella zoster virus infections in immunocompromised hosts. A review of the natural history and management. Am J Med. 1988; 85(2A):68–73.
11. Johnson JL, Amzat R, Martin N. Herpes zoster ophthalmicus. Prim Care. 2015; 42(3):285–303.
12. Vrcek I, Choudhury E, Durairaj V. Herpes zoster ophthalmicus: a review for the internist. Am J Med. 2017; 130(1):21–6.
13. Yawn BP, Wollan PC, St Sauver JL, Butterfield LC. Herpes zoster eye complications: rates and trends. Mayo Clin Proc. 2013; 88(6):562–70.
14. Shrestha M, Chen A. Modalities in managing postherpetic neuralgia. Korean J Pain. 2018; 31(4):235–43.
15. Kalogeropoulos CD, Bassukas ID, Moschos MM, Tabbara KF. Eye and periocular skin involvement in herpes zoster infection. Med Hypothesis Discov Innov Ophthalmol. 2015; 4(4):142–56.
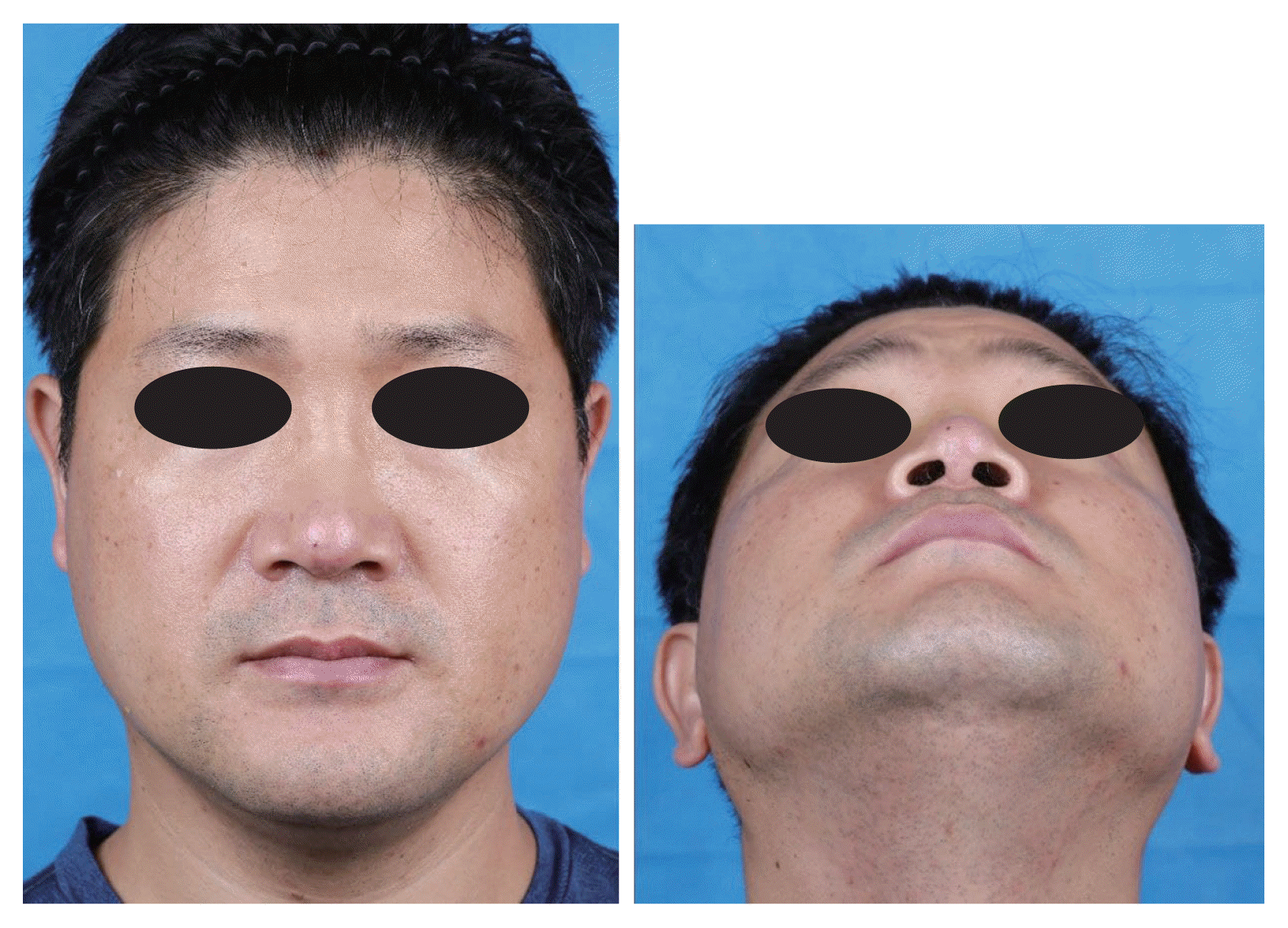
Fig. 1
Frontal and basal views of the patient five days after the first visit show erythematous patch with eschar around the nasal tip and erythematous crusted plaque around the right medial eyebrow.
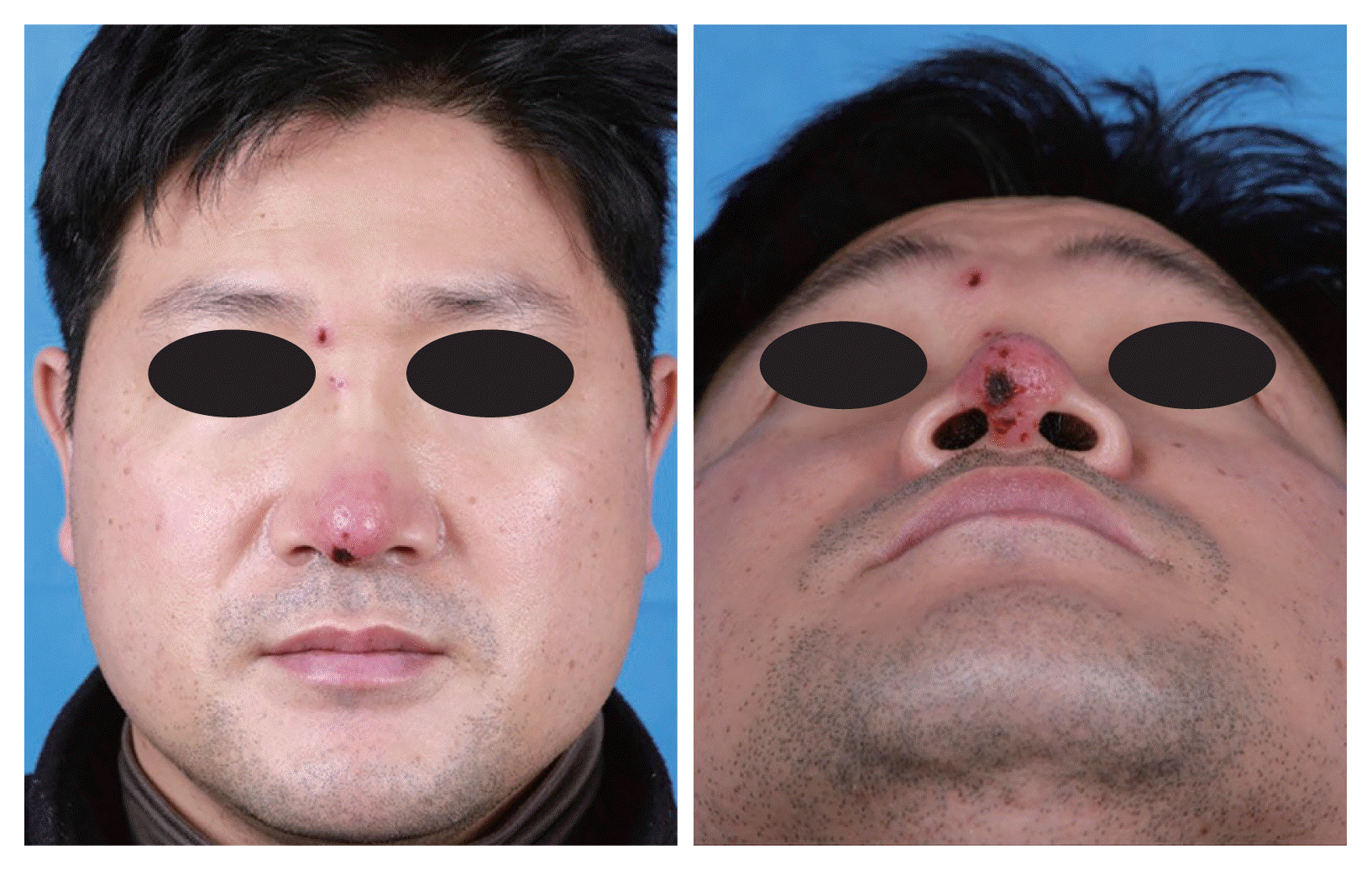
Fig. 2
Intraoperative endoscopic images show mild hyperemia in the vestibular skin (left internal valve area).
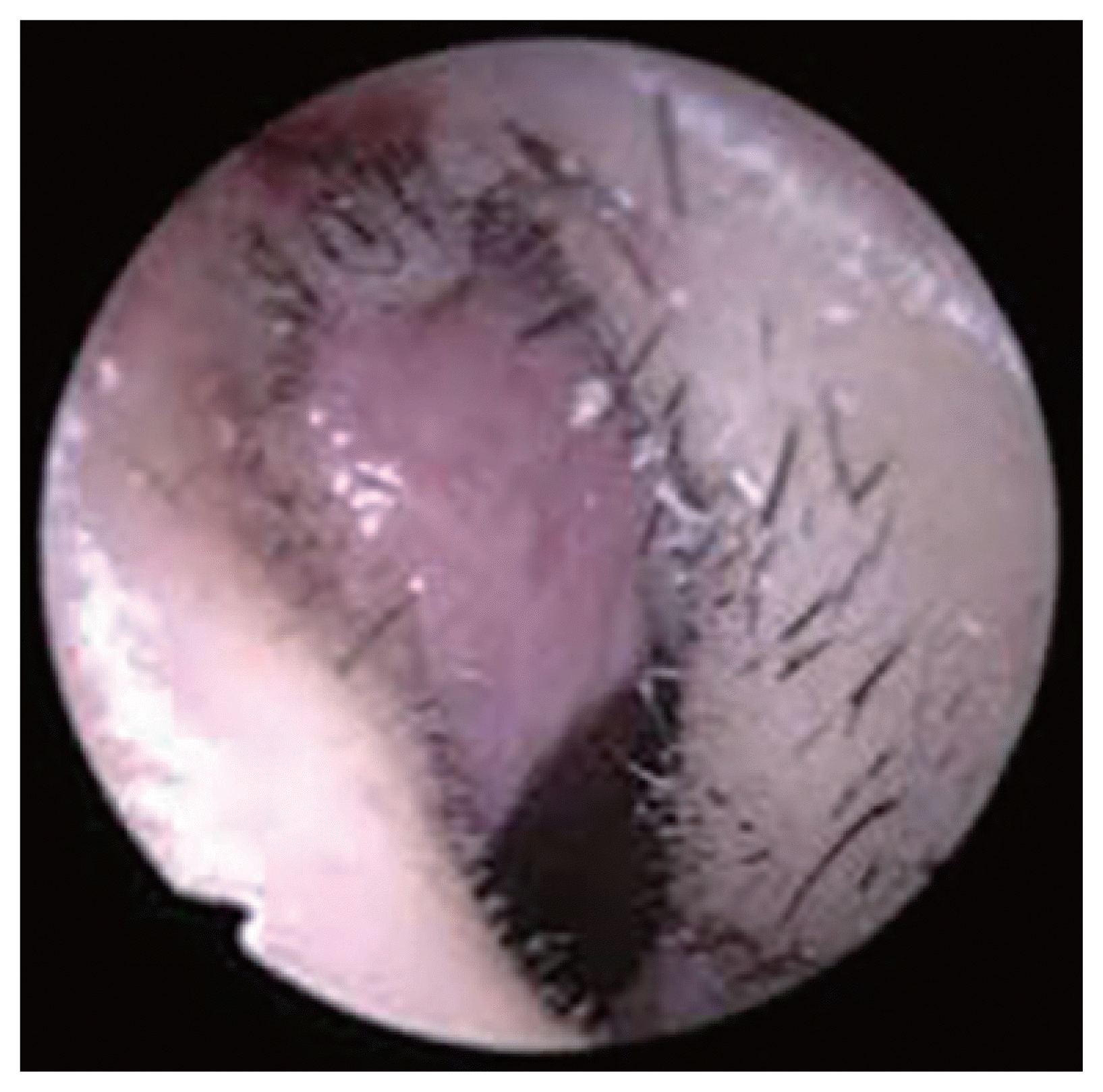
Fig. 3
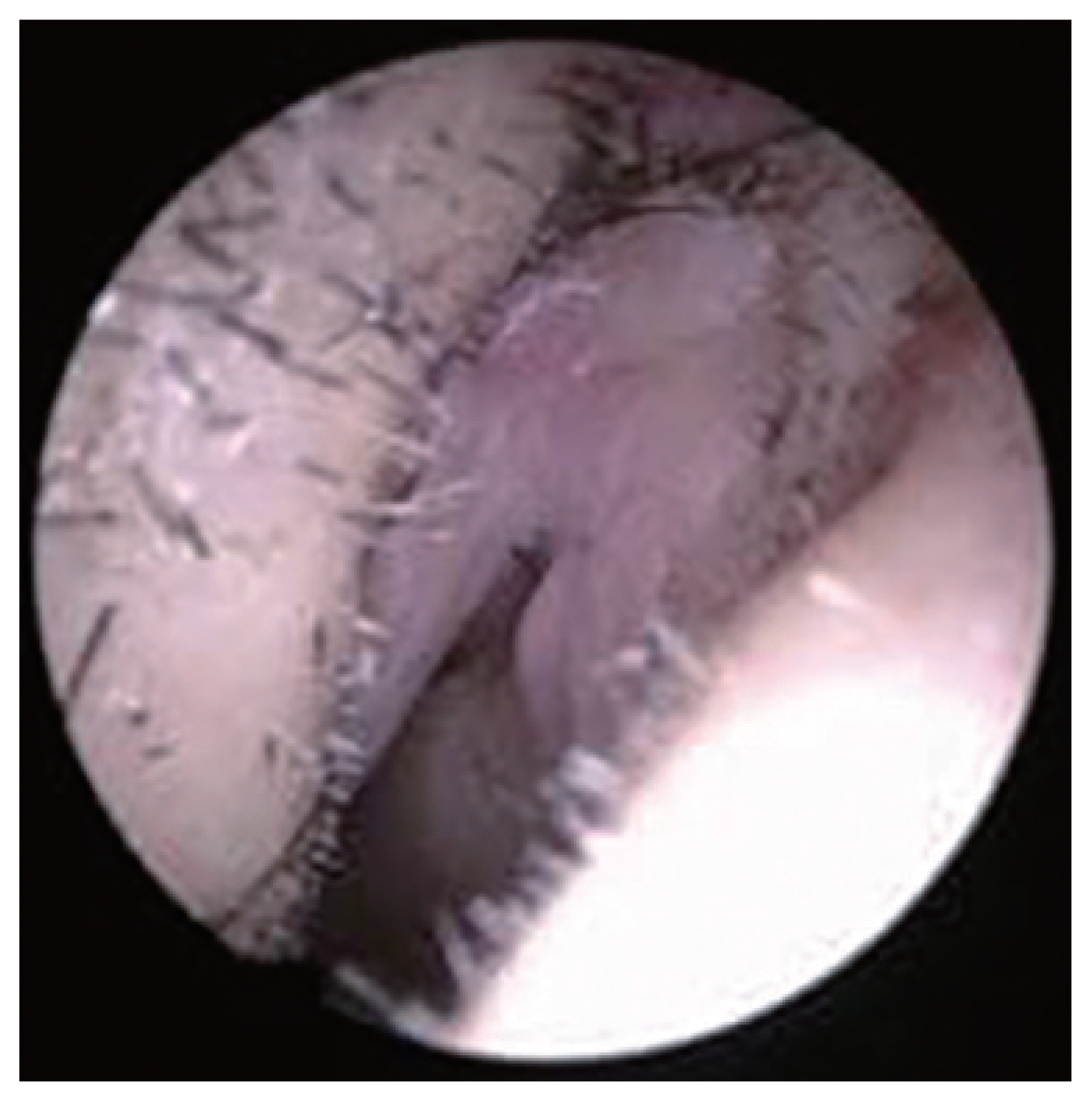
Intraoperative endoscopic images show mild hyperemia in the vestibular skin (right internal valve area).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download