Abstract
Budd-Chiari syndrome (BCS) is defined by the obstruction of the hepatic venous outflow between the small hepatic veins and the junction of the inferior vena cava (IVC) with the right atrium. BCS with IVC obstruction occasionally progresses to hepatocellular carcinoma (HCC). Here, we report the case of a patient with HCC arising from a cirrhotic liver with BCS, in whom the hepatic portion of the IVC was obstructed, and who had a favorable outcome with a multidisciplinary approach and IVC balloon angioplasty.
Budd-Chiari syndrome (BCS) is characterized by hepatic venous flow obstruction. Decreased venous outflow can arise at any point between the small hepatic veins and the junction of the inferior vena cava (IVC) with the right atrium of the heart.1 There are two forms of BCS: primary hepatic vein obstruction, also called classic BCS, and inferior vena cava of the hepatic portion obstruction (IVCO).2 Classic BCS is common in Europe and America. It is mostly associated with hereditary and acquired hypercoagulable states.3-5 IVCO is usually found in East Asia and South Africa. The etiology has not been clarified yet.6-8
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the third leading cause of cancer-related deaths.9,10 Hepatitis B and C, chronic alcohol consumption, non-alcoholic fatty liver disease, and primary biliary cirrhosis are the major risk factors for HCC.11-15 Some studies have shown that BCS, especially with IVC obstruction, occasionally progresses to HCC.2,7,16,17
In this report, we present a case of HCC arising from a cirrhotic liver with BCS, treated using a multidisciplinary approach and balloon angioplasty. HCC was treated using a multi-disciplinary approach, including transarterial chemoembolization (TACE), lenvatinib, and external beam radiation therapy (EBRT). Balloon angioplasty was performed on the collapsed IVC.
This case report is described in accordance with the CARE guidelines (available at https://www.care-statement.org/).
A 57-year-old woman presented to our hospital with epigastric pain that had persisted for 2 days. She had been diagnosed with liver cirrhosis of unknown etiology 25 years prior and had a history of gallbladder stones.
The results of the initial blood tests were as follows: white blood cell count, 10,520/µL; hemoglobin, 14.9 g/dL; platelet count, 125,000/µL; total bilirubin, 7.80 mg/dL; direct bilirubin, 5.06 mg/dL; aspartate aminotransferase, 303 U/L; alanine aminotransferase, 290 U/L; alkaline phosphatase, 321 U/L; and gamma-glutamyl transferase, 722 U/L. Serological tests for hepatitis B and C and autoimmune markers were negative. The levels of alpha-fetoprotein (AFP) and protein induced by the absence of vitamin K or antagonist-II (PIVKA-II) were 9,455.2 ng/mL and 10,916 mAU/mL, respectively. Contrast-enhanced abdominal computed tomography revealed heterogeneous enhancing lesions suspected of HCC in the right hepatic lobe (segments 4 and 7) and an abrupt cutoff point at the IVC. IVC venography showed collapse of the hepatic segment of the IVC, suggesting BCS (Fig. 1). The initial magnetic resonance imaging (MRI) of the liver demonstrated two tumors with the longest diameter of approximately 4.6 cm and 1.7 cm, respectively, with typical radiological features, including hypervascularity in the arterial phase, washout in the transitional phase, and hepatobiliary phase defect, with restricted diffusion (Fig. 2).
The patient was diagnosed with BCS based on the imaging findings that showed an occluded IVC in the hepatic segment. Liver cirrhosis (LC) and HCC were diagnosed based on the imaging findings. Because all the viral and autoimmune markers were negative and the patient had no history of alcohol consumption, we assumed that the etiology of LC and HCC was BCS. There was no evidence of distant metastasis on further imaging examinations. With two tumors (4.6 cm at S4, and 1.7 cm at S7), no regional lymph node or distant metastasis, Child-Pugh Class B, and performance status 0, the stage of HCC was intermediate stage (B) through Barcelona Clinic Liver Cancer (BCLC) staging. Based on the modified Union for International Cancer Control (UICC) staging, the HCC was classified as stage III.
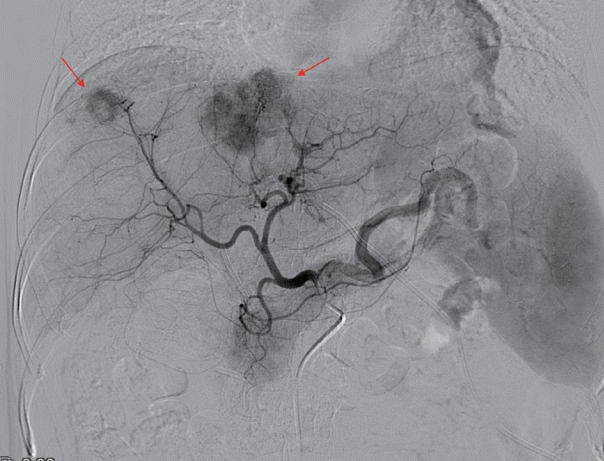
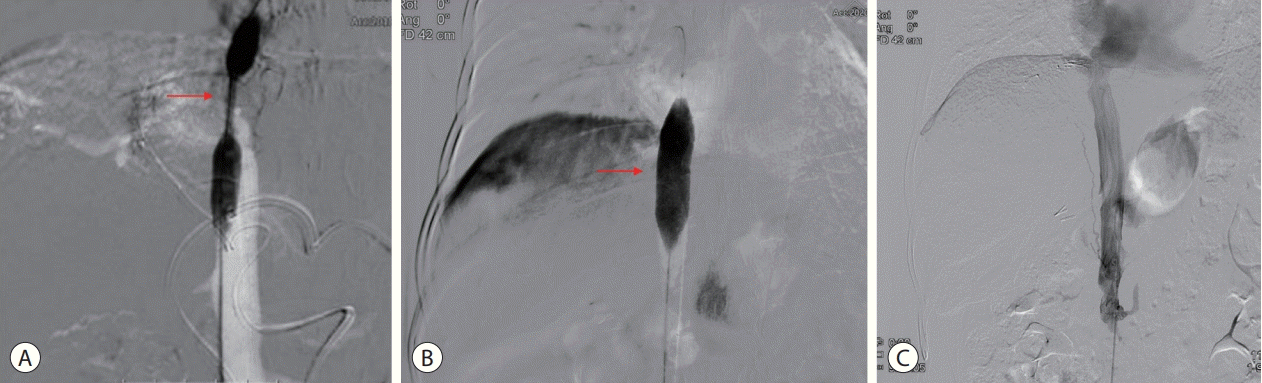
We first recommended that our patient undergo liver transplantation (LT) after downstaging by TACE, but she refused to be managed with LT. Conventional TACE was performed to treat HCC (Fig. 3), and IVC venography with balloon angioplasty was performed to treat BCS (Fig. 4).
Within 3 years of diagnosis, the patient underwent TACE and IVC balloon angioplasty six times for the treatment of HCC with BCS. The 2nd to 6th TACE were performed 2, 5, 8, 17, and 28 months after the first TACE. The 2nd, 3rd, 4th TACE procedures were performed because of insufficient response to treatment. Although a partially treated residual tumor at S4 was observed, the remaining viable tumor component was noted at the S4 lesion. After the 4th TACE, no viable portion of the treated HCC was noted, and remission of the disease was considered through imaging studies. However, recurrence occurred 9 months after the 4th TACE. The 5th and 6th TACE were performed because of recurrence. Newly noted lesions at S7 and S4 were seen before the 5th and 6th TACE, respectively. A follow-up imaging study showed the obliteration of intrahepatic arteries supplying the lesion; therefore, repetition of TACE was technically difficult as the tumor was supplied by collateral vessels. The patient refused additional TACE because of severe post-embolization syndrome. She had persistent high fever and severe abdominal pain after TACE. Consequently, the patient received lenvatinib for 8 weeks. However, AFP and PIVKA-II levels increased from 490 ng/mL to 625 ng/mL and from 18 mAU/mL to 430 mAU/mL, respectively, and the viable tumor size at the S4 dome increased from 5.5 cm to 7 cm. We evaluated lesion progression using mRECIST and considered it to be progressive disease with a 27.2% increase in tumor size. Subsequently, we performed EBRT. As a result, AFP and PIVKA-II levels were significantly decreased from 9,455.2 ng/mL to 5.03 ng/mL, from 10,916 mAU/mL to 26 mAU/mL, respectively. No viable lesions were observed on the last liver MRI performed 39 months after initial treatment (Fig. 5).
BCS is defined by obstruction of the hepatic venous outflow at any level between the small hepatic veins and the junction of the IVC with the right atrium.18 Classic BCS and IVCO are the two types of BCS. IVCO often progresses to HCC, whereas classic BCS seldom does.2 Likewise, in our case, the patient was also found to have HCC with obstruction of the hepatic portion of the IVC. BCS is a rare disorder, and its progression to HCC is rare. The prevalence of HCC in BCS differs from region to region. The IVCO type is frequently reported in Asia and Africa, but is rare in America and Europe.19 The pathogenesis of HCC with BCS has not yet been clarified. Long-term congestion caused by the reduction of the hepatic venous outflow can lead to fibrosis and it may contribute progressing to cirrhosis and HCC.16 Management for BCS involves anticoagulation, decompression with vascular intervention, portosystemic shunting or liver transplantation.20 Angioplasty in BCS aims to relieve hepatic congestion and restore hepatic blood flow. This can be achieved using balloon angioplasty or stenting.5,21
In our patient, HCC arising from LC with BCS with a collapsed hepatic segment of the IVC was treated with a multidisciplinary approach, such as TACE, lenvatinib, and EBRT. After administering six rounds of TACE, lenvatinib for 8 weeks, and EBRT at 36 months after the initial therapy, AFP and PIVKA-II levels returned to the normal range and remained there for 7 months. No new tumors were noted on the latest liver MRI performed 39 months after the initial treatment.
In conclusion, we presented a case of HCC arising from a cirrhotic liver with BCS. The patient was successfully treated with a multidisciplinary approach. Simultaneously, multiple rounds of balloon angioplasty were performed to enhance the liver perfusion. BCS may also be one of the causes of HCC, and clinicians should always be aware of this.
Notes
Ethics Statement
This study was approved by the Ethics Committee of the Catholic University of Korea (Approval No. KC22ZISI0624), and written informed consent was obtained from the patient.
Funding Statement
This work was also supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2021R1C1C1005844).
REFERENCES
1. Goel RM, Johnston EL, Patel KV, Wong T. Budd-Chiari syndrome: investigation, treatment and outcomes. Postgrad Med J. 2015; 91:692–697.
2. Matsui S, Ichida T, Watanabe M, Sugitani S, Suda T, Takahashi T, et al. Clinical features and etiology of hepatocellular carcinoma arising in patients with membranous obstruction of the inferior vena cava: in reference to hepatitis viral infection. J Gastroenterol Hepatol. 2000; 15:1205–1211.
3. Darwish Murad S, Plessier A, Hernandez-Guerra M, Fabris F, Eapen CE, Bahr MJ, et al. Etiology, management, and outcome of the Budd-Chiari syndrome. Ann Intern Med. 2009; 151:167–175.
4. Valla DC. Budd-Chiari syndrome and veno-occlusive disease/sinusoidal obstruction syndrome. Gut. 2008; 57:1469–1478.
5. DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study Liver Diseases. Vascular disorders of the liver. Hepatology. 2009; 49:1729–1764.
6. Shin SH, Chung YH, Suh DD, Shin JW, Jang MK, Ryu SH, et al. Characteristic clinical features of hepatocellular carcinoma associated with Budd-Chiari syndrome: evidence of different carcinogenic process from hepatitis B virus-associated hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2004; 16:319–324.
7. Shrestha SM, Okuda K, Uchida T, Maharjan KG, Shrestha S, Joshi BL, et al. Endemicity and clinical picture of liver disease due to obstruction of the hepatic portion of the inferior vena cava in Nepal. J Gastroenterol Hepatol. 1996; 11:170–179.
8. Qi X, Zhang C, Han G, Zhang W, He C, Yin Z, et al. Prevalence of the JAK2V617F mutation in Chinese patients with Budd-Chiari syndrome and portal vein thrombosis: a prospective study. J Gastroenterol Hepatol. 2012; 27:1036–1043.
9. Oliveri RS, Wetterslev J, Gluud C. Hepatocellular carcinoma. Lancet. 2012; 380:470. author reply 470-1.
10. Sung PS. Crosstalk between tumor-associated macrophages and neighboring cells in hepatocellular carcinoma. Clin Mol Hepatol. 2022; 28:333–350.
11. Lambert MP, Paliwal A, Vaissière T, Chemin I, Zoulim F, Tommasino M, et al. Aberrant DNA methylation distinguishes hepatocellular carcinoma associated with HBV and HCV infection and alcohol intake. J Hepatol. 2011; 54:705–715.
12. Stickel F, Hampe J. Genetic determinants of alcoholic liver disease. Gut. 2012; 61:150–159.
13. Krawczyk M, Bonfrate L, Portincasa P. Nonalcoholic fatty liver disease. Best Pract Res Clin Gastroenterol. 2010; 24:695–708.
14. Liang Y, Yang Z, Zhong R. Primary biliary cirrhosis and cancer risk: a systematic review and meta-analysis. Hepatology. 2012; 56:1409–1417.
15. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011; 365:1118–1127.
16. Gwon D 2nd, Ko GY, Yoon HK, Sung KB, Kim JH, Lee SS, et al. Hepatocellular carcinoma associated with membranous obstruction of the inferior vena cava: incidence, characteristics, and risk factors and clinical efficacy of TACE. Radiology. 2010; 254:617–626.
17. Shrestha SM. Liver cirrhosis and hepatocellular carcinoma in hepatic vena cava disease, a liver disease caused by obstruction of inferior vena cava. Hepatol Int. 2009; 3:392–402.
18. Sung PS, Oh JS, Choi JI. Acute Budd-Chiari syndrome with thrombotic thrombocytopenia after BNT162b2 mRNA vaccination. Liver Int. 2022; 42:1447–1448.
19. Kew MC, McKnight A, Hodkinson J, Bukofzer S, Esser JD. The role of membranous obstruction of the inferior vena cava in the etiology of hepatocellular carcinoma in Southern African blacks. Hepatology. 1989; 9:121–125.
20. Martens P, Nevens F. Budd-Chiari syndrome. United European Gastroenterol J. 2015; 3:489–500.
21. Valla D, Hadengue A, el Younsi M, Azar N, Zeitoun G, Boudet MJ, et al. Hepatic venous outflow block caused by short-length hepatic vein stenoses. Hepatology. 1997; 25:814–819.
Figure 1.
(A) Initial contrast-enhanced abdominal cT shows collapse of the hepatic portion of the IVc (arrow). (B) Initial IVc venography shows occlusion of the hepatic portion of the IVc (arrows). cT, computed tomography; IVc, inferior vena cava.
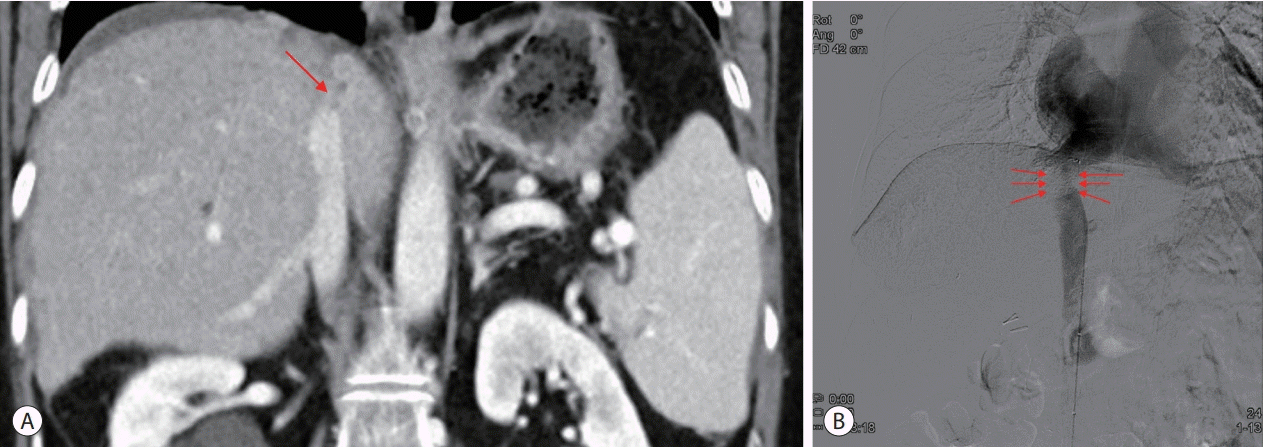
Figure 2.
Initial magnetic resonance imaging of the liver demonstrated two tumors with the longest diameter of approximately 4.6 cm and 1.7 cm in S4 and S7, respectively, with typical radiological features. (A) Hypervascularity in arterial phase (left), washout in transitional phase (middle), and defect in hepatobiliary phase (right) after dynamic contrast enhancement (arrows). (B) High signal intensity in high b value image (left), diffusion restriction (right) in diffusion weighted image (arrows).
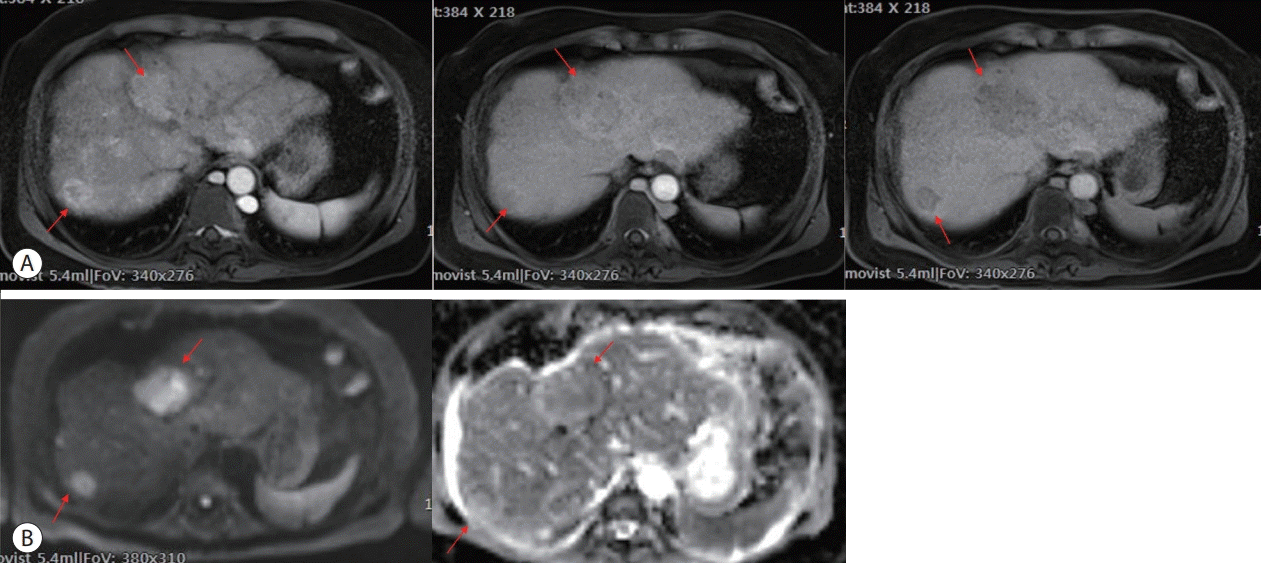
Figure 3.
Initial transarterial chemoembolization being performed to treat hepatocellular carcinoma (arrows).




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download