Abstract
Advances in our knowledge of the molecular characteristics of hepatocellular carcinoma (HCC) have enabled significant progress in the detection and therapeutic prediction of HCC. As a non-invasive alternative to tissue biopsy, liquid biopsy examines circulating cellular components such as exosomes, nucleic acids, and cell-free DNA found in body fluids (e.g., urine, saliva, ascites, and pleural effusions) and provides information about tumor characteristics. Technical advances in liquid biopsy have led to the increasing adoption of diagnostic and monitoring applications for HCC. This review summarizes the various analytes, ongoing clinical trials, and case studies of United States Food and Drug Administrationapproved in vitro diagnostic applications for liquid biopsy, and provides insight into its implementation in managing HCC.
Hepatocellular carcinoma (HCC) is the most common liver cancer and the fourth leading cause of cancer-related deaths worldwide in 2020.1 The 5-year survival rate of patients with HCC is 15%, making it the second most lethal tumor after pancreatic cancer.2 In South Korea, the incidence of HCC was 21.2 per 100,000 person-years (crude incidence) and 13.9 per 100,000 person-years (age-standardized incidence) in 2018.3,4 Moreover, its mortality rate in 2019 was 20.6%,5 which is the second highest mortality rate among the most common cancers in South Korea in 2021.
The etiologies of HCC include hepatitis B virus (HBV)/hepatitis C virus (HCV) infection, non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis, and alcohol abuse.4,6 Each year, HCC reportedly affects 2-7% of patients with active HCV- or HBV-related cirrhosis worldwide.7,8 NAFLD has recently become a leading cause of HCC and is predicted to affect more than 10 million adults in the United States by 2030.9 The prevalence of NAFLD is approximately 25%,10 and the annual incidence of HCC among patients with NAFLD was 1.06% in a large cohort study conducted in the United States.11 Based on the evidence described above, the Clinical Practice Guidelines12 of the Korean Association for the Study of the Liver recommend that high-risk patients undergo biannual HCC screening by ultrasound imaging, with or without alpha-fetoprotein (AFP) levels. In a long-term follow-up study on the efficacy of HCC screening, the survival rate was better in patients who underwent regular assessment than in those who did not.13 However, ultrasound screening for HCC demonstrated a sensitivity of 63%, which was even lower (47%) than that for patients with cirrhosis.14
Advances in our knowledge of the molecular characteristics of HCC, combined with the development of new liquid biopsy technologies, have enabled significant progress in the early detection and therapeutic monitoring of HCC using blood. As a non-invasive alternative to tissue biopsy, liquid biopsy provides information about tumor characteristics through assays involving body fluids. Liquid biopsy has advantages over traditional tissue biopsy: sample collection is minimally invasive and liquid biopsy also allows repeated sampling, which enables real-time monitoring of the molecular characteristics. Moreover, tissue biopsy is affected by intra-tumor heterogeneity, whereas liquid biopsy can provide a heterogeneous molecular profile.15
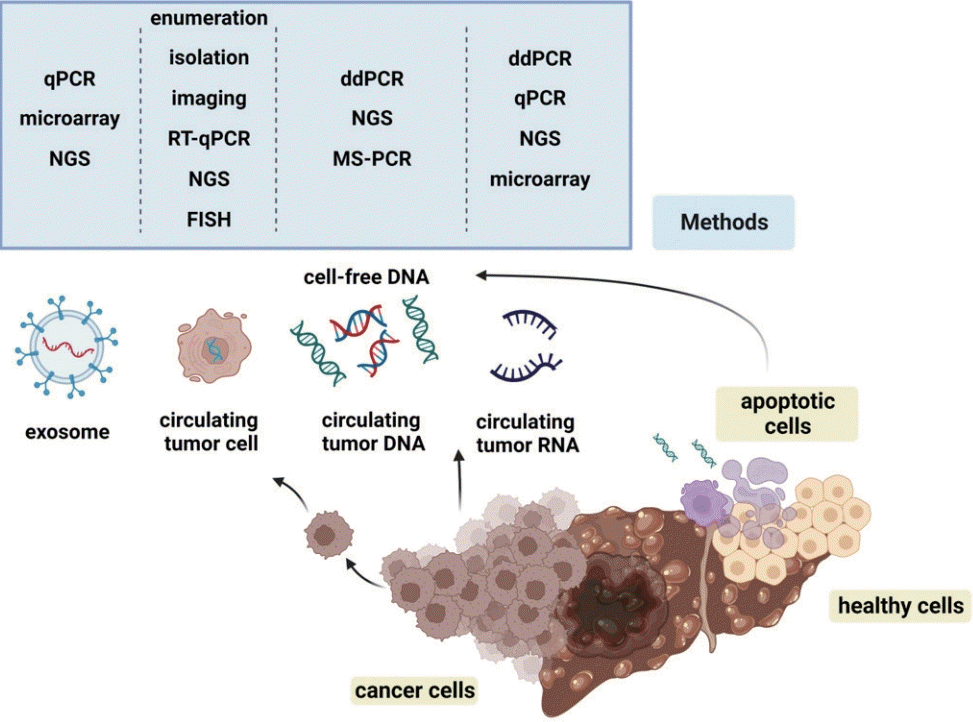
Circulating cellular components, such as exosomes, nucleic acids, cell-free DNA (cfDNA), cell-free RNA (cfRNA), and cancer tumor cells (CTCs), found in body fluids (e.g., urine, saliva, ascites, and pleural effusions16) of cancer patients are used for tumor detection and monitoring. Exosomes are extracellular vesicles released from cells and are known to carry bioactive molecules that facilitate cancer growth.17 CTCs are cancer cells that are detached from the primary tumor and are known for their role in metastasis.18 cfDNA and ctDNA are DNA fragments that exist in the naked form during cellular processes such as necrosis and apoptosis. Similarly, cfRNA is shed into the blood from both cancerous and noncancerous cells (Fig. 1).
Current liquid biopsy applications include early diagnosis, minimal residual disease detection, therapeutic selection guidance, and prognostic prediction.19 The most common application for liquid biopsy is companion diagnostics, which is a test that provides the applicability of a therapeutic drug to a specific person (Fig. 2).20 The United States Food and Drug Administration (FDA)-approved liquid biopsy tests include CancerSEEK, Guardant360® CDx, FoundationOne® Liquid CDx, Cologuard, and EpiColon. CancerSEEK detects mutations in 16 cancer-related genes and eight proteins to identify the presence of early stage cancers. Whereas CancerSEEK is designed to detect multiple cancers, Cologuard and EpiColon specifically detect colorectal cancer (CRC). Guardant360® CDx and FoundationOne® Liquid CDx are companion diagnostic tests that detect panels of mutations and can be used to guide treatment.21 Guardant360® CDx is designed to identify mutations in non-small cell lung cancer. In contrast, FoundationOne® Liquid CDx detects a high blood tumor mutational burden, high microsatellite instability, and tumor fraction values in solid tumors.
Commercially available approaches to liquid biopsy, in either LDT or FDA-approved kit.21,22 are used in current clinical practice for tumor tests23 and tests for circulating tumor cells or DNA. This review focuses on ctDNA and cfDNA testing with a brief description of other approaches. The biomarkers used in ctDNA tests include DNA mutations, copy number alterations, transcriptome signatures, proteins, DNA methylation, and metabolic abnormalities. This review describes the modalities of liquid biopsies as well as their applications in the diagnosis of HCC and monitoring of therapeutic efficacy in affected patients.
cfDNA is a fractionated portion of circulating nucleic acids (150-200 base pairs) that originates from various cellular events (e.g., apoptosis, necrosis, pyroptosis, and autophagy).24 cfDNA was first discovered by Mandel and Metais in 1948.25 Subsequently, Leon et al.26 revealed that elevated levels of cfDNA were present in the serum of patients with cancer. The cfDNA concentration in patients with cancer varies widely from 0 ng/mL to >1,000 ng/mL, whereas the mean concentration is 30 ng/mL in healthy individuals.27,28 Concordantly, higher cfDNA concentrations have been observed in patients with advanced cancer than in those with early stage cancer. Notably, cfDNA concentrations correlated with tumor size.29 cfDNA concentrations vary according to the cancer type. Patients with liver cancer were shown to have the highest cfDNA levels among various cancers, suggesting that HCC is an appropriate type of cancer for detection via cfDNA analysis.30 As the amount of cfDNA in the blood reflects complex tumor biology (e.g., tumor burden, tumor metabolism, and apoptosis) and non-cancerous blood cell death associated with high inflammation, identifying the cfDNA source is essential for accurately assessing cancer. The characteristics of ctDNAs have been extensively studied to improve the identification of tumor-derived cfDNAs. ctDNA constitutes 0.01-90% of cfDNA31 and has a half-life of 1-2.4 hours.32 The length of ctDNA is 50-150 bp, which is shorter than that of most cfDNAs.33,34 The presence of ctDNA in cfDNA can be identified through assays that detect tumorrelated biomarkers with high specificity. The concentration, integrity, mutations, and methylation of cfDNA are among the tumor-related biomarkers that can be measured using liquid biopsy. These tumor biomarkers are detected by various means, including droplet digital polymerase chain reaction (PCR), quantitative PCR (qPCR), whole-genome sequencing, whole-exome sequencing, targeted sequencing, and methylation-sensitive high-resolution melting analyses.35 Although each of these technologies has unique advantages and limitations, a common requirement for liquid biopsy is the ability to detect low levels of ctDNA within cfDNA (usually with a variant allele frequency <1%).
Genomic alterations such as single-nucleotide variations, copy number variations, fragmentation, and viral integration have been used as diagnostic biomarkers.36 Analytical platforms for mutation detection include qPCR, targeted sequencing, whole-genome sequencing, and whole-exome sequencing. qPCR and targeted sequencing are limited to known or designed mutations; thus, they may not detect clonal evolution involving mutations absent from the assay design.37 Whole-genome sequencing and whole-exome sequencing can be expanded to cover unknown mutations; however, they are costly and require manual review for variant analysis.38 The standard approach in clinical practice involves the exploration of mutations using droplet digital PCR or targeted sequencing.39 The advantages of gene-based genomic alteration biomarkers include the detection of mutant allele fractions ≥0.001%,40 provision of druggable target information, and facilitation of real-time patient monitoring. A notable challenge in mutation-based ctDNA detection is that genetic alterations primarily occur in the late stages of cancer.30 Consequently, early detection of cancer using mutations generally has low sensitivity. In addition, mutations do not provide information concerning the tissue of origin.41 In addition to mutations, various genomic events such as genomic rearrangements, mutational signatures, and copy number changes can be used as liquid biopsy biomarkers. The DELFI method (i.e., DNA evaluation of fragments for early interception) can detect abnormalities in cfDNA through the analysis of fragmentation patterns.42 Compared to non-cancer cfDNA, cancer cfDNA has a higher fragmentation profile.43 In addition, fragmentation analyses provide tissue-specific information required for diagnosis using blood. However, the main applications of cfDNA mutations are to guide clinical treatment, monitor therapeutic responses, and predict the risk of getting cancer.36 For example, HCC patients with RAS mutations have a higher clinical response to refametinib combined with sorafenib.44 Another example is oncogenic mutation of the Wnt/-catenin (CTNNB1) pathway. β-catenin mutations are associated with T cell rejection and immunotherapy resistance,45 suggesting adverse outcomes in patients treated with immune checkpoint inhibitors.
Significant epigenetic and genetic modifications have been observed in early preneoplastic liver tissues, which are presumed to drive tumorigenesis.46 Global hypomethylation and hypermethylation of promoters and CpG islands are common phenomena in diverse cancers. Well-established HCC methylation biomarkers include septin 9 (SEPT9), vimentin (VIM), fibulin 1 (FBLN1), tissue factor pathway inhibitor 2 (TFPI2), G protein-coupled bile acid receptor 1 (TGR5), homeobox A1 (HOXA1), empty spiracle homeobox 1 (EMX1), TSPY-like 5 (TSPYL5), metallothionein 1M (MT1M), and metallothionein 1G (MTIG).29,47
Currently, epigenetic biomarkers are used in two commercialized liquid biopsy products for HCC, Oncoguard Liver (Current Procedural Terminology Test Code: 81599) and HelioLiver (Current Procedural Terminology Test Code: 16222). Oncoguard Liver uses an algorithm based on multiple markers, such as sex, age, HCC methylation (HOXA1, EMX1, and TSPYL5), and AFP.48 During clinical validation for detecting early stage HCC, Oncoguard Liver demonstrated a sensitivity of 72% and a specificity of 88%49 in a study population that included 159 HCC samples and 250 control samples. HelioLiver uses a pre-specified diagnostic algorithm that measures cfDNA methylation levels in 28 genes (77 CpG sites), three protein tumor markers (AFP, AFP‐L3%, and des-gamma-carboxy prothrombin), and patient demographic characteristics (age and sex). HelioLiver demonstrated sensitivities of 85% for HCC at any stage and 76% for early stage HCC, with a specificity of 91%50 in a study population that included 122 HCC samples and 125 control samples.
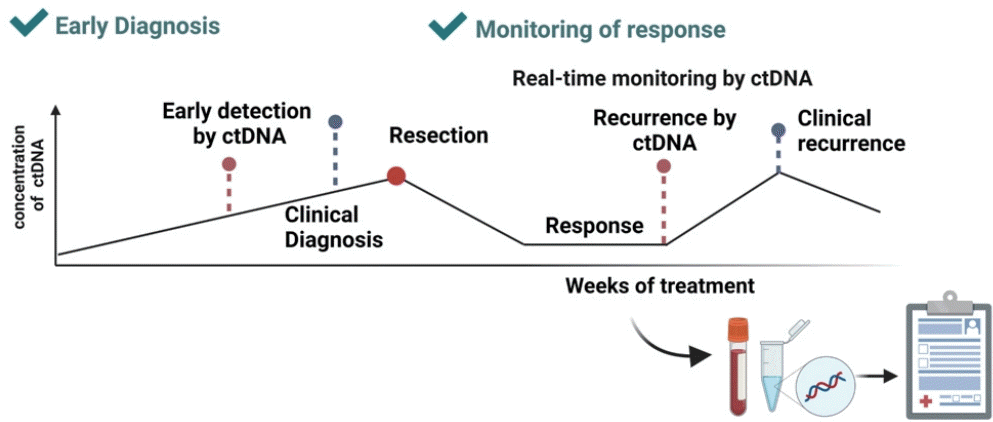
Several studies have monitored cfDNA concentration in relation to treatment outcomes. In a previous study, cfDNA was quantified on admission and after treatment. Of the 48 patients, 17 were controlled without complications, 16 experienced recurrence, and 15 developed post-operative complications. The investigators measured the copy numbers of beta-2-microglobulin (B2M) and peptidyl-prolyl cis-trans isomerase A (PPIA) in cfDNA. Before surgery, the cfDNA concentrations did not differ between the groups; after surgery, the PPIA copy number was higher among patients who developed complications.51 Analysis of resectable liver metastases of CRC revealed that the presence of postoperative ctDNA in RAS mutant–positive patients is significantly associated with a lower recurrence-free survival rate.52 Similarly, a study monitored changes in cfDNA, ctDNA (telomerase reverse transcriptase [TERT] mutations), and AFP before (D1) and after (D+2 and M+1) transarterial chemoembolization (TACE) treatment. Treatment responders showed a significant increase in the baseline and post-treatment (M+1) concentrations of cfDNA and ctDNA, whereas the AFP level did not correctly predict tumor response.53 Moreover, the mutation burden of ctDNA and cfDNA in patients with recurrence is higher.54
An investigator-initiated phase 2 study of pembrolizumab immunological response evaluation (INSPIRE) evaluated the genomic and immunological landscape of peripheral blood from solid tumors after pembrolizumab treatment. The results showed that genomic features from the blood, including fold changes in the numbers of CD4+ T cells and Ki67+ programmed cell death protein 1 (PD-1)+ CD8+ T cells, were significantly correlated with the clinical response to pembrolizumab.55
Another application of liquid biopsy is the detection of multiple cancers with a single test that can provide information concerning the tissue of origin. Some obstacles to this application include the potential for inaccurate tissue-of-origin predictions owing to alterations not exclusive to any specific cancer. The analysis of epigenetic changes is superior to the study of genetic changes in predicting tumor tissues of origin.56 CancerSEEK is an FDA-approved in vitro diagnostic test that examines 16 gene-based and eight protein-based biomarkers for ovarian, stomach, liver, esophageal, pancreatic, colorectal, breast, and lung cancers. Clinical validation studies have demonstrated 33-98% sensitivity, with a specificity of 99% and a median tissue of origin accuracy of 63%.57
Galleri is a pan-cancer test for >50 types of cancer developed by Grail, LLC. Grail enrolled >134,000 participants in the Galleri.58,59 In a circulating cell-free genome atlas study, the Galleri test detected signals from 50 types of cancer with a specificity of 99.5% and tissue of origin accuracy of 89%; sensitivity varied according to the type of cancer, ranging from 11.2% to 93.5%.60 Galleri has not acquired FDA approval and has been commercialized as a laboratory-developed test. Grail’s laboratory is certified by the Clinical Laboratory Improvement Amendments of 1988. The extensive clinical research conducted by Grail aimed to prove clinical effectiveness by separating clinical designs according to the population with a long follow-up period and the clinical utility of Galleri for screening for multiple cancers.
Other analytes for liquid biopsy include cfRNA, exosomes, and circulating tumor cells. Various types of cfRNAs are present in the blood, including messenger RNA (mRNA), microRNA (miRNA), long noncoding RNA (lncRNA), piwiinteracting RNA, and transfer RNA.61 cfRNA is produced during necrotic and apoptotic processes and is then released into the bloodstream. Notably, analyses of cfRNA can correctly identify the tumor tissue of origin using cell-type decomposition or cell-type-specific RNA markers.62 Transcriptome-wide characterization of cfRNAs in cancer has identified mRNAs specific to lung and breast cancers. Analysis of cfRNA can improve the detection rate in patients with low levels of ctDNA and allow for the prediction of tumor tissue of origin and the identification of cancer subtypes.63 MicroRNAs (e.g., miR-1, miR-122, miR-21, miR-26a, miR-29a, miR-155, miR-96, and miR-99a) have been associated with survival in patients with HCC.64-66
Exosomes are extracellular vesicles with phospholipid bilayers 30-150 nm in size. Since the identification of exosomes in the late 1980s, they have been shown to carry diverse cargoes, including long noncoding RNA, mRNAs, and proteins. In addition to their diverse cargo transport abilities, exosomes have good stability in all body fluids, low immunogenicity, and biocompatibility. Thus, they have attracted attention for use in various applications (e.g., liquid biopsy and drug delivery).67 Exosome targets in HCC include proteins, mRNA, miRNAs, lncRNAs, circular RNA, and DNA.68 Exosome levels have been correlated with tumor size and aggressiveness,69,70 and exosomes have been reported to transport signaling molecules involved in angiogenesis and tumorigenesis. In a currently available diagnostic test, the combined analysis of extracellular vesicle mRNA showed a sensitivity of 94% and specificity of 75%.71 On the other hand, combined analyses of extracellular vesicle miRNA and AFP showed a sensitivity of 86% and specificity of 88%.72 Currently, cfRNA and exosome analyses have not yet been implemented in clinical practice and require assay standardization and appropriate guidelines for in vitro diagnostic governance.
CTCs are defined as tumor cells from primary or metastasized tumors that leave the site of origin to the peripheral blood system.73 Since they were first described in 1869, CTCs have often been described as seeds of metastatic tumors.74 Several studies have focused on the use of CTCs to monitor disease progression and predict prognosis.75 In addition to the epithelial to mesenchymal transition phenotype, morphological changes in the blood to withstand biological events such as shear stress and immune attacks add metastatic potential.76,77 Thus, CTCs are separated and captured using various techniques based on their altered biophysical characteristics.78 Separation using physical properties includes sizebased and density-based separation.79 On the other hand, separation based on biological properties uses immunoaffinity and immunomagnetic separation. To this end, epithelial markers (EpCAM/CK8,18,1980,81), mesenchymal markers (vimentin and Twist82), and HCC-specific markers (GPC3 and ASGPR) were used to capture CTCs in patients.83,84 Because of the association of CTCs with their role in metastasis, numerous studies have been conducted to monitor and predict therapeutic interventions. EpCAM+ CTCs have been shown to have a worse prognosis and a higher rate of recurrence in HCC.85,86 Consistently, increases in CTCs were correlated with an increased risk of tumor recurrence and extrahaptic metastasis in liver resection and TACE.87-90 The advantage of CTCs is their further utilization in single-cell, transcriptome, and genomic analyses to identify therapeutic targets and molecular characteristics.91 The drawback is the heterogeneity of biomarkers, lack of sensitivity due to 20-35% of HCC patients with EpCAM expression,92, and low signal-to-noise ratio in early stage disease.93,94 Nevertheless, continuous studies using CTCs in monitoring and predicting therapeutic responses would elucidate the metastatic mechanism of HCC as the previous researches had.
Although different analytes and methods have been used in liquid biopsies, the search for suitable biomarkers is still being actively pursued in many clinical studies. A review of ongoing clinical studies can provide insights into the implementation of liquid biopsies in clinical settings. For this purpose, we performed a search for clinical trials involving HCC through June 23, 2022, on clinicalTrials.gov, using the terms “liquid biopsy OR cfDNA OR ctDNA”, which yielded 39 results (16 recruiting, four not yet recruiting, six completed, six with unknown status, two terminated, three active/not recruiting, and two enrolling by invitation only). Of the 39 clinical trials, ten focused on diagnostic feasibility, seven on changes in ctDNA as an outcome for a drug, five on biomarker exploration (i.e., observational analysis), and 17 on therapeutic monitoring (Supplementary Table 1). Therapeutic responses were monitored after sequential sorafenib-regorafenib treatment, transplantation, TACE, transarterial radioembolization, liver resection, immune checkpoint inhibitor treatments, combined immunotherapy, targeted therapy (PD-1 or sorafenib), and tyrosine kinase inhibitor treatments. In addition, ctDNA analysis has been included as an additional outcome measure in interventional clinical trials of new treatments such as GT90001+nivolumab combination therapy, chiauranib (phases 1 and 2), pembrolizumab or KEYTRUDA® for pediatric HCC, nivolumab+yttrium Y-90 combination therapy, itacitinib (phase Ib), and other open-label studies (Supplementary Table 2). The incorporation of ctDNA analyses in the early phases of clinical trials indicates the importance of stratifying patient groups at the molecular level. Active clinical evaluations of liquid biopsy include a study of the diagnostic accuracy of SEPT9 promoter methylation, the diagnostic accuracy of gender, age, AFPL3%, AFP, des-gamma-carboxy prothrombin (GALAD) score, and a study without disclosed biomarkers (Supplementary Table 3). Additionally, to develop an adequate liquid biopsy for HCC, studies on novel biomarkers, actionable alterations, and concordant ctDNA markers with tumor tissues are actively pursued (Supplementary Table 4).
Since no FDA-approved liquid biopsy for HCC diagnosis exists, the most well-established cancer diagnostic test, Cologuard, developed by Exact Sciences, is discussed to understand the clinical study considerations required for approval. Cologuard is the first and only FDA-approved stool-based DNA screening test for CRC. The safety and effectiveness of Cologuard were assessed in a prospective, cross-sectional, multicenter, pivotal study (“Multi-Target Colorectal Cancer Screening Test for the Detection of Colorectal Advanced Adenomatous Polyps and Cancer: DeeP-C Study”). This study involved 12,776 patients aged 45 years and older with an average risk of CRC who were enrolled at 90 sites from June 2011 to February 2013. Patient stool samples were analyzed using the Cologuard test and a fecal immunochemical test (FIT). After 90 days, all enrolled patients underwent colonoscopy. The results of the Cologuard and FIT analyses were compared with colonoscopic and histopathological findings. The overall sensitivity and specificity of Cologuard were 92% and 87%, respectively. In this clinical study, Cologuard demonstrated robust performance in two co-primary endpoint analyses: sensitivity in terms of patients diagnosed with CRC (>65% sensitivity for CRC) and specificity in terms of patients without CRC or advanced neoplasia (AN) (>85% specificity for AN). Secondary endpoint analyses showed that Cologuard was non-inferior to the FIT in terms of sensitivity for CRC and superior to the FIT in terms of sensitivity for advanced adenoma (McNemar test P-value=0.0018). In addition, they conducted in silico simulations to show clinical utility in large population settings. Cologuard screens adults 45 or older at an average risk of CRC and does not replace diagnostic colonoscopy.95
Based on the above clinical studies, validation of liquid biopsy for HCC should consider how new diagnostic methods could be incorporated into the current HCC diagnosis workflow, including the identification of appropriate clinical support. According to the 2022 HCC clinical guidelines, the diagnostic workflow proceeds from the surveillance of patients at high risk for HCC to first-line imaging, principal imaging, and ancillary/second-line imaging.11 Current clinical studies suggest that liquid biopsies should be incorporated into surveillance and ancillary imaging (e.g., positron emission tomography-magnetic resonance imaging+ctDNA). Additionally, trial participants should be representative of the target population to prove their clinical effectiveness and utility.96 For example, patients at high risk for HCC (e.g., patients with chronic HBV infection, chronic HCV infection, or cirrhosis) should be included in clinical trials if the intended indication involves the surveillance of patients at high risk for HCC. Moreover, considerations of the primary and secondary endpoints to prove the effectiveness of the test should include a comparison with current standard methods (AFP assessment and ultrasound) and clinical sensitivity/specificity boundaries. Furthermore, risk-benefit analyses should be included, as in the Cologuard study, to determine whether a particular benefit justifies the risk of the new diagnostic method.
Many challenges must be addressed for liquid biopsy to become a standard component of clinical practice, including ensuring cost-effectiveness, evaluating clinical utility, establishing regulations, and building a testing laboratory infrastructure. The lack of pre-analytical and analytical standards due to variability in liquid biopsy analytes and the absence of cost-effectiveness validation studies have hindered the incorporation of liquid biopsy into clinical practice.97 The pre-analytical factors to consider include the selection of blood collection tubes, timing of sample transit, use of plasma or serum, guidelines for storage conditions, methods for purification/quantification of cfDNA, and protocols for sample preparation.98 Analytical factors to explore include the lack of performance standards in validation studies (e.g., limits of detection/quantitation/blanks), the need for reproducibility, the need to reduce interference, and the need to establish thresholds of analytical sensitivity and specificity.
The United States and China are conducting large-scale clinical trials to promote the incorporation of liquid biopsy into clinical practice. The factors involved in their approaches include well-established laboratory-based tests, robust funding environments, and the potential for large-cohort studies. As liquid biopsy platforms range from PCR to next-generation sequencing, there is a need for collaboration among regulatory bodies, diagnostics development companies, and other research facilities to establish pre-analytical and analytical standards that consider platforms and specimens. Basic research institutions and diagnostics development companies should confirm biomarker feasibility in independent settings, assess whether each biomarker is sufficiently representative of multiple molecular types of cancer, and clearly define the potential representative populations for biomarkers. The relevant standards should be developed through numerous clinical trials with input from patients, healthcare providers, and grant coordinators. Finally, although most clinical trial outcome data are unavailable for acceptable reasons, data sharing and communication among scientists is essential for incorporating this novel technology into clinical practice. The primary concern of these stakeholders should be the scientific advancement of next-generation diagnostics and the well-being of patients who may benefit from liquid biopsy.
Notes
Ethics Statement
This article is fully based on the articles that were already published and did not involve additional patient participants. Therefore, IRB approval was not necessary.
Funding Statement
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI22C0147).
Supplementary Material
Supplementary data can be found with this article online https://doi.org/10.17998/jlc.2022.09.08.
REFERENCES
1. International Agency for Research on Cancer. Estimated number of deaths in 2020, World, both sexes, all ages (excl. NMSC) [Internet]. Geneva (CH): World Health Organization;[cited 2022 Aug 1]. Available from: https://gco.iarc.fr/today/online-analysistable?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=39&type=1&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=0&include_nmsc_other=1.
2. Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, et al. Annual report to the nation on the status of cancer, 1975-2014, featuring survival. J Natl Cancer Inst. 2017; 109:djx030.
3. National Cancer Center. Annual report of cancer statistics in Korea in 2018 [Internet]. Goyang (KR): National Cancer Center;[cited 2022 Aug 1]. Available from: https://ncc.re.kr/cancerStatsView.ncc?bbsnum=558&searchKey=total&searchValue=&pageNum=1.
4. Chon YE, Jeong SW, Jun DW. Hepatocellular carcinoma statistics in South Korea. Clin Mol Hepatol. 2021; 27:512–514.
5. Statistics Korea. Preliminary results of birth and death statistics in 2021 [Internet]. Daejeon (KR): Statistics Korea;[cited 2022 Aug 1]. Available from: http://kostat.go.kr/portal/eng/pressReleases/8/10/index.board?bmode=read&bSeq=&aSeq=417980&pageNo=1&rowNum=10&navCount=10&currPg=&searchInfo=&sTarget=title&sTxt=.
6. Cha C, Dematteo RP. Molecular mechanisms in hepatocellular carcinoma development. Best Pract Res Clin Gastroenterol. 2005; 19:25–37.
7. Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981; 2:1129–1133.
8. Niederau C, Lange S, Heintges T, Erhardt A, Buschkamp M, Hürter D, et al. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology. 1998; 28:1687–1695.
9. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018; 67:123–133.
10. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Metaanalytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016; 64:73–84.
11. Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018; 155:1828–1837.e2.
12. Korean Association for the Study of the Liver. KASL clinical practice guidelines: management of chronic hepatitis B. Clin Mol Hepatol. 2016; 22:18–75.
13. Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004; 130:417–422.
14. Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a metaanalysis. Gastroenterology. 2018; 154:1706–1718. e1.
15. Xu RH, Wei W, Krawczyk M, Wang W, Luo H, Flagg K, et al. Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat Mater. 2017; 16:1155–1161.
16. Macías M, Alegre E, Díaz-Lagares A, Patiño A, Pérez-Gracia JL, Sanmamed M, et al. Liquid biopsy: from basic research to clinical practice. Adv Clin Chem. 2018; 83:73–119.
17. Tai YL, Chen KC, Hsieh JT, Shen TL. Exosomes in cancer development and clinical applications. Cancer Sci. 2018; 109:2364–2374.
18. Yang C, Xia BR, Jin WL, Lou G. Circulating tumor cells in precision oncology: clinical applications in liquid biopsy and 3D organoid model. Cancer Cell Int. 2019; 19:341.
19. García-Pardo M, Makarem M, Li JJN, Kelly D, Leighl NB. Integrating circulating-free DNA (cfDNA) analysis into clinical practice: opportunities and challenges. Br J Cancer. 2022; 127:592–602.
20. U.S. Food and Drug Administration. Companion diagnostics [Internet]. Silver Spring (MD): U.S. Food and Drug Administration;[cited 2022 Aug 1]. Available from: https://www.fda.gov/medicaldevices/in-vitro-diagnostics/companion-diagnostics.
21. U.S. Food and Drug Administration. List of cleared or approved companion diagnostic devices (in vitro and imaging tools) [Internet]. Silver Spring (MD): U.S. Food and Drug Administration;[cited 2022 Aug 1]. Available from: https://www.fda.gov/medicaldevices/in-vitro-diagnostics/list-cleared-or-approved-companiondiagnostic-devices-in-vitro-and-imaging-tools.
22. The ASCO Post. The evolution of liquid biopsy in cancer care [Internet]. Huntington (NY): The ASCO Post;[cited 2022 Sep 2]. Available from: https://ascopost.com/issues/october-10-2021/theevolution-of-liquid-biopsy-in-cancer-care/.
23. Testing.com. Liquid biopsy [Internet]. Seattle (WA): Testing.com;[cited 2022 Aug 1]. Available from: https://www.testing.com/tests/liquid-biopsy/.
24. Grabuschnig S, Bronkhorst AJ, Holdenrieder S, Rosales Rodriguez I, Schliep KP, Schwendenwein D, et al. Putative origins of cell-free DNA in humans: a review of active and passive nucleic acid release mechanisms. Int J Mol Sci. 2020; 21:8062.
25. Mandel P, Metais P. Nuclear acids in human blood plasma. C R Seances Soc Biol Fil. 1948; 142:241–243.
26. Leon SA, Shapiro B, Sklaroff DM, Yaros MJ. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 1977; 37:646–650.
27. Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011; 11:426–437.
28. Thierry AR, El Messaoudi S, Gahan PB, Anker P, Stroun M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016; 35:347–376.
29. Alunni-Fabbroni M, Rönsch K, Huber T, Cyran CC, Seidensticker M, Mayerle J, et al. Circulating DNA as prognostic biomarker in patients with advanced hepatocellular carcinoma: a translational exploratory study from the SORAMIC trial. J Transl Med. 2019; 17:328.
30. van der Pol Y, Mouliere F. Toward the early detection of cancer by decoding the epigenetic and environmental fingerprints of cell-free DNA. Cancer Cell. 2019; 36:350–368.
31. Corcoran RB, Chabner BA. Application of cell-free DNA analysis to cancer treatment. N Engl J Med. 2018; 379:1754–1765.
32. Mohme M, Riethdorf S, Pantel K. Circulating and disseminated tumour cells - mechanisms of immune surveillance and escape. Nat Rev Clin Oncol. 2017; 14:155–167.
33. Underhill HR, Kitzman JO, Hellwig S, Welker NC, Daza R, Baker DN, et al. Fragment length of circulating tumor DNA. PLoS Genet. 2016; 12:e1006162.
34. Mouliere F, Chandrananda D, Piskorz AM, Moore EK, Morris J, Ahlborn LB, et al. Enhanced detection of circulating tumor DNA by fragment size analysis. Sci Transl Med. 2018; 10:eaat4921.
35. Lyu X, Tsui YM, Ho DW, Ng IO. Liquid biopsy using cell-free or circulating tumor DNA in the management of hepatocellular carcinoma. Cell Mol Gastroenterol Hepatol. 2022; 13:1611–1624.
36. Piñero F, Dirchwolf M, Pessôa MG. Biomarkers in hepatocellular carcinoma: diagnosis, prognosis and treatment response assessment. Cells. 2020; 9:1370.
37. Nakamura Y, Shitara K. Development of circulating tumour DNA analysis for gastrointestinal cancers. ESMO Open. 2020; 5(Suppl 1):e000600.
38. Koboldt DC. Best practices for variant calling in clinical sequencing. Genome Med. 2020; 12:91.
39. Keller L, Belloum Y, Wikman H, Pantel K. Clinical relevance of blood-based ctDNA analysis: mutation detection and beyond. Br J Cancer. 2021; 124:345–358.
40. Ou CY, Vu T, Grunwald JT, Toledano M, Zimak J, Toosky M, et al. An ultrasensitive test for profiling circulating tumor DNA using integrated comprehensive droplet digital detection. Lab Chip. 2019; 19:993–1005.
41. Gao Q, Zeng Q, Wang Z, Li C, Xu Y, Cui P, et al. Circulating cell-free DNA for cancer early detection. Innovation (Camb). 2022; 3:100259.
42. Cristiano S, Leal A, Phallen J, Fiksel J, Adleff V, Bruhm DC, et al. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature. 2019; 570:385–389.
43. Mathios D, Johansen JS, Cristiano S, Medina JE, Phallen J, Larsen KR, et al. Detection and characterization of lung cancer using cellfree DNA fragmentomes. Nat Commun. 2021; 12:5060.
44. Lim HY, Heo J, Choi HJ, Lin CY, Yoon JH, Hsu C, et al. A phase II study of the efficacy and safety of the combination therapy of the MEK inhibitor refametinib (BAY 86-9766) plus sorafenib for Asian patients with unresectable hepatocellular carcinoma. Clin Cancer Res. 2014; 20:5976–5985.
45. Li X, Xiang Y, Li F, Yin C, Li B, Ke X. WNT/β-catenin signaling pathway regulating T cell-inflammation in the tumor microenvironment. Front Immunol. 2019; 10:2293.
46. Hlady RA, Zhou D, Puszyk W, Roberts LR, Liu C, Robertson KD. Initiation of aberrant DNA methylation patterns and heterogeneity in precancerous lesions of human hepatocellular cancer. Epigenetics. 2017; 12:215–225.
47. Han LY, Fan YC, Mu NN, Gao S, Li F, Ji XF, et al. Aberrant DNA methylation of G-protein-coupled bile acid receptor Gpbar1 (TGR5) is a potential biomarker for hepatitis B Virus associated hepatocellular carcinoma. Int J Med Sci. 2014; 11:164–171.
48. Chalasani NP, Ramasubramanian TS, Bhattacharya A, Olson MC, Edwards V DK, Roberts LR, et al. A novel blood-based panel of methylated DNA and protein markers for detection of early-stage hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2021; 19:2597–2605. e4.
49. Chalasani NP, Porter K, Bhattacharya A, Book AJ, Neis BM, Xiong KM, et al. Validation of a novel multitarget blood test shows high sensitivity to detect early stage hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2022; 20:173–182. e7.
50. Lin N, Lin Y, Xu J, Liu D, Li D, Meng H, et al. A multi-analyte cellfree DNA-based blood test for early detection of hepatocellular carcinoma. Hepatol Commun. 2022; 6:1753–1763.
51. Rosen AW, Gögenur M, Paulsen IW, Olsen J, Eiholm S, Kirkeby LT, et al. Perioperative changes in cell-free DNA for patients undergoing surgery for colon cancer. BMC Gastroenterol. 2022; 22:168.
52. Bolhuis K, van ‘t Erve I, Mijnals C, Delis-Van Diemen PM, Huiskens J, Komurcu A, et al. Postoperative circulating tumour DNA is associated with pathologic response and recurrence-free survival after resection of colorectal cancer liver metastases. EBioMedicine. 2021; 70:103498.
53. Sefrioui D, Verdier V, Savoye-Collet C, Beaussire L, Ghomadi S, Gangloff A, et al. Circulating DNA changes are predictive of disease progression after transarterial chemoembolization. Int J Cancer. 2022; 150:532–541.
54. Li Z, Xiao D, Li X, Zhan P, Wang J, Zhang H. Early recurrence detected in hepatocellular carcinoma patients after transcatheter arterial chemoembolization treatment with plasma cell-free DNA. Eur J Gastroenterol Hepatol. 2019; 31:885–892.
55. Clouthier DL, Lien SC, Yang SYC, Nguyen LT, Manem VSK, Gray D, et al. An interim report on the investigator-initiated phase 2 study of pembrolizumab immunological response evaluation (INSPIRE). J Immunother Cancer. 2019; 7:72.
56. Köhler F, Rodríguez-Paredes M. DNA methylation in epidermal differentiation, aging, and cancer. J Invest Dermatol. 2020; 140:38–47.
57. Lennon AM, Buchanan AH, Kinde I, Warren A, Honushefsky A, Cohain AT, et al. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science. 2020; 369:e. abb9601.
58. GRAIL. Clinical expertise [Internet]. San Francisco (CA): GRAIL;[cited 2022 Aug 1]. Available from: https://grail.com/clinicalexpertise/.
59. Liu S, Wang J. Current and future perspectives of cell-free DNA in liquid biopsy. Curr Issues Mol Biol. 2022; 44:2695–2709.
60. Klein EA, Richards D, Cohn A, Tummala M, Lapham R, Cosgrove D, et al. Clinical validation of a targeted methylation-based multicancer early detection test using an independent validation set. Ann Oncol. 2021; 32:1167–1177.
61. Danielson KM, Rubio R, Abderazzaq F, Das S, Wang YE. High throughput sequencing of extracellular RNA from human plasma. PLoS One. 2017; 12:e0164644.
62. Vorperian SK, Moufarrej MN; Tabula Sapiens Consortium, Quake SR. Cell types of origin of the cell-free transcriptome. Nat Biotechnol. 2022; 40:855–861.
63. Larson MH, Pan W, Kim HJ, Mauntz RE, Stuart SM, Pimentel M, et al. A comprehensive characterization of the cell-free transcriptome reveals tissue- and subtype-specific biomarkers for cancer detection. Nat Commun. 2021; 12:2357.
64. Köberle V, Kronenberger B, Pleli T, Trojan J, Imelmann E, PevelingOberhag J, et al. Serum microRNA-1 and microRNA-122 are prognostic markers in patients with hepatocellular carcinoma. Eur J Cancer. 2013; 49:3442–3449.
65. Xu Y, Bu X, Dai C, Shang C. High serum microRNA-122 level is independently associated with higher overall survival rate in hepatocellular carcinoma patients. Tumour Biol. 2015; 36:4773–4776.
66. Cho HJ, Kim SS, Nam JS, Kim JK, Lee JH, Kim B, et al. Low levels of circulating microRNA-26a/29a as poor prognostic markers in patients with hepatocellular carcinoma who underwent curative treatment. Clin Res Hepatol Gastroenterol. 2017; 41:181–189.
67. Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019; 88:487–514.
68. Chen W, Mao Y, Liu C, Wu H, Chen S. Exosome in hepatocellular carcinoma: an update. J Cancer. 2021; 12:2526–2536.
69. Whiteside TL. Tumor-derived exosomes and their role in cancer progression. Adv Clin Chem. 2016; 74:103–141.
70. Shen M, Di K, He H, Xia Y, Xie H, Huang R, et al. Progress in exosome associated tumor markers and their detection methods. Mol Biomed. 2020; 1:3.
71. Sun N, Lee YT, Zhang RY, Kao R, Teng PC, Yang Y, et al. Purification of HCC-specific extracellular vesicles on nanosubstrates for early HCC detection by digital scoring. Nat Commun. 2020; 11:4489.
72. Wang Y, Zhang C, Zhang P, Guo G, Jiang T, Zhao X, et al. Serum exosomal microRNAs combined with alpha-fetoprotein as diagnostic markers of hepatocellular carcinoma. Cancer Med. 2018; 7:1670–1679.
73. Ashworth TR. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Med J Aust. 1869; 14:146–147.
74. Recasens A, Munoz L. Targeting cancer cell dormancy. Trends Pharmacol Sci. 2019; 40:128–141.
75. Cui K, Ou Y, Shen Y, Li S, Sun Z. Clinical value of circulating tumor cells for the diagnosis and prognosis of hepatocellular carcinoma (HCC): a systematic review and meta-analysis. Medicine (Baltimore). 2020; 99:e22242.
76. Miller MC, Doyle GV, Terstappen LW. Significance of circulating tumor cells detected by the CellSearch system in patients with metastatic breast colorectal and prostate cancer. J Oncol. 2010; 2010:617421.
77. Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014; 158:1110–1122.
78. Ahn JC, Teng PC, Chen PJ, Posadas E, Tseng HR, Lu SC, et al. Detection of circulating tumor cells and their implications as a biomarker for diagnosis, prognostication, and therapeutic monitoring in hepatocellular carcinoma. Hepatology. 2021; 73:422–436.
79. Bankó P, Lee SY, Nagygyörgy V, Zrínyi M, Chae CH, Cho DH, et al. Technologies for circulating tumor cell separation from whole blood. J Hematol Oncol. 2019; 12:48.
80. Ou H, Huang Y, Xiang L, Chen Z, Fang Y, Lin Y, et al. Circulating tumor cell phenotype indicates poor survival and recurrence after surgery for hepatocellular carcinoma. Dig Dis Sci. 2018; 63:2373–2380.
81. Yu JJ, Xiao W, Dong SL, Liang HF, Zhang ZW, Zhang BX, et al. Effect of surgical liver resection on circulating tumor cells in patients with hepatocellular carcinoma. BMC Cancer. 2018; 18:835.
82. Li YM, Xu SC, Li J, Han KQ, Pi HF, Zheng L, et al. Epithelial-mesenchymal transition markers expressed in circulating tumor cells in hepatocellular carcinoma patients with different stages of disease. Cell Death Dis. 2013; 4:e831.
83. Court CM, Hou S, Winograd P, Segel NH, Li QW, Zhu Y, et al. A novel multimarker assay for the phenotypic profiling of circulating tumor cells in hepatocellular carcinoma. Liver Transpl. 2018; 24:946–960.
84. Yi B, Wu T, Zhu N, Huang Y, Yang X, Yuan L, et al. The clinical significance of CTC enrichment by GPC3-IML and its genetic analysis in hepatocellular carcinoma. J Nanobiotechnology. 2021; 19:74.
85. Noh CK, Wang HJ, Kim CM, Kim J, Yoon SY, Lee GH, et al. EpCAM as a predictive marker of tumor recurrence and survival in patients who underwent surgical resection for hepatocellular carcinoma. Anticancer Res. 2018; 38:4101–4109.
86. Zhou Y, Wang B, Wu J, Zhang C, Zhou Y, Yang X, et al. Association of preoperative EpCAM circulating tumor cells and peripheral Treg cell levels with early recurrence of hepatocellular carcinoma following radical hepatic resection. BMC Cancer. 2016; 16:506.
87. Guo W, Yang XR, Sun YF, Shen MN, Ma XL, Wu J, et al. Clinical significance of EpCAM mRNA-positive circulating tumor cells in hepatocellular carcinoma by an optimized negative enrichment and qRT-PCR-based platform. Clin Cancer Res. 2014; 20:4794–4805.
88. Ye X, Li G, Han C, Han Q, Shang L, Su H, et al. Circulating tumor cells as a potential biomarker for postoperative clinical outcome in HBV-related hepatocellular carcinoma. Cancer Manag Res. 2018; 10:5639–5647.
89. Teng PC, Agopian VG, Lin TY, You S, Zhu Y, Tseng HR, et al. Circulating tumor cells: A step toward precision medicine in hepatocellular carcinoma. J Gastroenterol Hepatol. 2022; 37:1179–1190.
90. Sun YF, Xu Y, Yang XR, Guo W, Zhang X, Qiu SJ, et al. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology. 2013; 57:1458–1468.
91. Zhang J, Quadri S, Wolfgang CL, Zheng L. New development of biomarkers for gastrointestinal cancers: from neoplastic cells to tumor microenvironment. Biomedicines. 2018; 6:87.
92. Abdelgawad IA. Epithelial cell adhesion molecule mRNA can be a potential marker to predict metastasis in hepatocellular carcinoma patients. Asian Pac J Cancer Prev. 2020; 21:861–866.
93. Fan JL, Yang YF, Yuan CH, Chen H, Wang FB. Circulating tumor cells for predicting the prognostic of patients with hepatocellular carcinoma: a meta analysis. Cell Physiol Biochem. 2015; 37:629–640.
94. Calabuig-Fariñas S, Jantus-Lewintre E, Herreros-Pomares A, Camps C. Circulating tumor cells versus circulating tumor DNA in lung cancer-which one will win? Transl Lung Cancer Res. 2016; 5:466–482.
95. Exact Sciences. Frequently asked questions [Internet]. Madison (WI): Cologuard;[cited 2022 Aug 1]. Available from: https://www.cologuardhcp.com/faq.
96. Li Q, He Z, Guo Y, Zhang H, George TJ, Hogan W, et al. Assessing the validity of a a priori patient-trial generalizability score using real-world data from a large clinical data research network: a colorectal cancer clinical trial case study. AMIA Annu Symp Proc. 2020; 2019:1101–1110.
97. Merker JD, Oxnard GR, Compton C, Diehn M, Hurley P, Lazar AJ, et al. Circulating tumor DNA analysis in patients with cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. J Clin Oncol. 2018; 36:1631–1641.
98. Fleischhacker M, Schmidt B. Pre-analytical issues in liquid biopsy – where do we stand? J Lab Med. 2020; 44:117–142.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download