Abstract
Background/Aim
Methods
Results
Notes
Ethics Statement
Patients were not required to provide informed consent because of the retrospective nature of the study and the use of fully de-identified data. The Institutional Review Board (IRB) of the Ajou University Hospital approved the study protocol (IRB No. AJIRB-MED-MDB-17-031).
Funding Statement
This research was supported by the Research Award of the Korean Liver Cancer Association (2021), the Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare, Republic of Korea (HI19C0872 and HR21C1003), and the Bio and Medical Technology Development Program of the National Research Foundation (NRF-2018M3A9E8023861 and NRF-2019R1C1C1004580) funded by the Korean government (MSIT).
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.
Author Contribution
Conceptualization: Kim SS, Ahn Y-H
Data curation: Cheong JY, Cho HJ, Lee H, Han JE
Formal analysis: Kim SS, Park B, Lee H, Ahn Y-H
Funding acquisition: Kim SS, Cheong JY
Investigation: Cheong JY, Cho HJ, Ahn Y-H, Han JE
Methodology: Kim SS, Park B
Project admission: Kim SS, Park B
Resource: Ahn Y-H, Lee H, Kim SS, Park B
Software: Park B, Lee H
Supervision: Kim SS, Park B
Validation: Park B, Lee H
Visualization: Lee H, Han JE
Writing-Original draft preparation: Ahn Y-H
Writing-Review and editing: Kim SS, Park B
Supplementary Material
REFERENCES
Figure 1.

Figure 2.
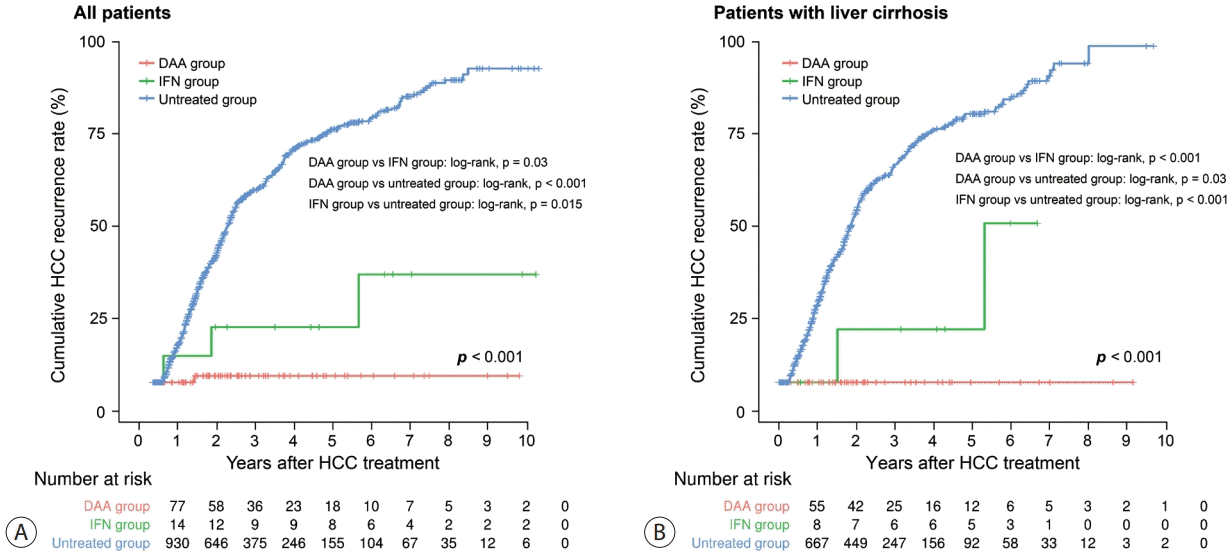
Table 1.
| Variable | DAA-treated group (n=77) | IFN-treated group (n=14) | Untreated group (n=930) | P-value |
|---|---|---|---|---|
| Age (years) | 65.6±8.8 | 58.5±7.5 | 68.5±8.8 | <0.001* |
| Male gender | 34 (41.2) | 11 (78.6) | 537 (57.7) | 0.017* |
| Diabetes mellitus | 35 (45.5) | 7 (50.0) | 341 (36.7) | 0.193 |
| Liver cirrhosis | 55 (71.4) | 8 (57.1) | 667 (71.7) | 0.457 |
| HCC recurrence | 1 (1.3) | 3 (21.4) | 507 (54.5) | <0.001* |
| HCC treatment modality | 0.196 | |||
| Resection | 3 (3.9) | 2 (14.3) | 102 (11.0) | |
| RFA | 74 (96.1) | 12 (85.7) | 826 (88.8) | |
| Resection+RFA | 2 (0.2) | |||
| DAA regimen | ||||
| Daclatasvir/asunaprevir | 46 (59.7) | |||
| Sofosbuvir/ribavirin | 25 (32.5) | |||
| Ledipasvir/sofosbuvir | 6 (7.8) | |||
| DAA regimen duration (weeks) | 13.1 (8.3-18.7) | |||
| <12 | 37 (48.1) | |||
| 12-24 | 40 (51.9) | |||
| IFN regimen duration (weeks) | 16.6 (5.3-42.6) | |||
| <12 | 7 (50.0) | |||
| 12-24 | 5 (35.7) | |||
| >24 | 2 (14.3) | |||
| Interval between HCC treatment to antiviral therapy, months | 14.3 (6.1-39.6) | 16.1 (2.6-22.1) | ||
| <3 | 12 (15.6) | 4 (28.6) | ||
| 3-6 | 7 (9.1) | 2 (14.3) | ||
| 6-12 | 15 (19.5) | 2 (14.3) | ||
| 12-24 | 16 (20.7) | 3 (21.4) | ||
| >24 | 27 (35.1) | 3 (21.4) |
Table 2.
| Variable | DAA-treated group (n=41) | IFN-treated group (n=2) | Untreated group (n=156) | P-value |
|---|---|---|---|---|
| Age (years) | 66.1±8.6 | 55.0±9.9 | 70.2±9.1 | 0.003* |
| Male gender | 18 (43.9) | 1 (50.0) | 88 (56.4) | 0.318 |
| Diabetes mellitus | 21 (51.2) | 1 (50.0) | 63 (40.4) | 0.413 |
| Liver cirrhosis | 30 (73.2) | 1 (50.0) | 117 (75.0) | 0.62 |
| HCC recurrence | 1 (2.4) | 0 (0.0) | 30 (19.2) | 0.013* |
| HCC treatment modality | 1 | |||
| Resection | 3 (1.9) | |||
| RFA | 41 (100.0) | 2 (100.0) | 153 (98.1) | |
| DAA regimen | ||||
| Daclatasvir/asunaprevir | 24 (58.5) | |||
| Sofosbuvir/ribavirin | 12 (29.3) | |||
| Ledipasvir/sofosbuvir | 5 (12.2) | |||
| DAA regimen duration (weeks) | 16.1 (13.1-21.1) | |||
| <12 | 14 (34.1) | |||
| 12-24 | 27 (65.9) | |||
| IFN regimen duration (weeks) | 10.3 (7.8-10.2) | |||
| <12 | 2 (100.0) | |||
| Interval between HCC treatment to antiviral therapy, months | 6.4 (2.9-11.5) | 6.1 (5.8-6.4) | ||
| <3 | 12 (29.3) | |||
| 3-6 | 7 (17.1) | 1 (50.0) | ||
| 6-12 | 13 (31.7) | 1 (50.0) | ||
| 12-24 | 9 (21.9) |
Table 3.
| Modality |
Univariate Cox |
Multivariate Cox* |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| DAA vs. untreated | 0.039 | 0.005-0.274 | 0.001† | 0.037 | 0.005-0.261 | <0.001† |
| DAA‡ vs. untreated | 0.042 | 0.006-0.303 | 0.001† | 0.040 | 0.006-0.289 | 0.001† |
| DAA§ vs. untreated | 0.052 | 0.007-0.371 | 0.003† | 0.050 | 0.007-0.354 | 0.003† |
| DAA or IFN vs. untreated | 0.089 | 0.029-0.279 | <0.001† | 0.086 | 0.027-0.268 | <0.001† |
| DAA or IFN‡ vs. untreated | 0.098 | 0.032-0.307 | <0.001† | 0.094 | 0.029-0.293 | <0.001† |
| DAA or IFN§ vs. untreated | 0.117 | 0.038-0.368 | <0.001† | 0.111 | 0.035-0.346 | <0.001† |
Table 4.
| Modality |
Univariate Cox |
Multivariate Cox* |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| DAA vs. untreated | 0.042 | 0.006-0.297 | 0.002† | 0.044 | 0.006-0.313 | 0.002† |
| DAA‡ vs. untreated | 0.047 | 0.007-0.336 | 0.002† | 0.049 | 0.007-0.349 | 0.003† |
| DAA§ vs. untreated | 0.060 | 0.008-0.427 | 0.004† | 0.063 | 0.009-0.451 | 0.006† |
| DAA or IFN vs. untreated | 0.060 | 0.015-0.243 | <0.001† | 0.066 | 0.016-0.266 | <0.001† |
| DAA or IFN‡ vs. untreated | 0.034 | 0.005-0.241 | <0.001† | 0.037 | 0.005-0.263 | <0.001† |
| DAA or IFN§ vs. untreated | 0.042 | 0.006-0.300 | 0.002† | 0.047 | 0.007-0.334 | 0.002† |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download