Abstract
The Fontan operation is performed in patients with a single ventricle. As the systemic venous return is directly connected to the pulmonary circulation during this procedure, chronic hepatic congestion is induced, leading to Fontan-associated liver disease (FALD) including liver cirrhosis and hepatocellular carcinoma (HCC). In this report, we present a case of HCC diagnosed in a patient who underwent the Fontan operation 30 years ago. The patient underwent regular surveillance for FALD, which revealed a 4 cm-sized hepatic mass with elevated serum alpha-fetoprotein. After surgical treatment, there was no evidence of HCC recurrence during 3 years of follow-up. As the risk of HCC and Fontan-associated liver cirrhosis increases with the duration elapsed since the operation, regular surveillance should be emphasized. Serial follow-up of serum alpha-fetoprotein levels and abdominal imaging are necessary to achieve early and accurate diagnosis of HCC in post-Fontan patients.
Since the Fontan procedure was first performed to correct tricuspid atresia by Fontan and Baudet in 1971,1 it has been modified for application in patients with single-ventricle physiology due to complex congenital heart diseases. The direct connection of systemic venous return to the pulmonary arteries in the Fontan circulation allows passive filling of the pulmonary circulation without the use of a pump. Therefore, high central venous pressure and low cardiac output due to limited preload are inevitable in patients who undergo the Fontan operation.2,3
As shown in Fig. 1, the hemodynamic characteristics of the Fontan circulation result in chronic hepatic congestion, causing liver cirrhosis and hepatocellular carcinoma (HCC).4,5 However, little is known about the risk of HCC after the Fontan procedure, and there are currently no consensus guidelines for surveillance of Fontan-associated liver disease (FALD).
Here, we present a case of HCC diagnosed in a patient who underwent Fontan operation 30 years ago. This case report demonstrates the importance of regular surveillance for liver disease following the Fontan procedure. This case report is described in accordance with the CARE guidelines (available at https://www.care-statement.org/).
A 35-year-old man was referred with a hepatic mass detected on computed tomography (CT) imaging at Seoul National University Hospital (SNUH). He was born with a single ventricle combined with pulmonary stenosis and a secundum atrial septal defect. The patient underwent left modified Blalock–Taussig shunt and Fontan operation at the ages of three and six, respectively. He was subsequently asymptomatic until 20 years of age, when he visited SNUH with worsening dyspnea on exertion and cyanosis. Conversion Fontan operation with mitral valve closure was performed at the age of 20 years.
The patient received regular surveillance of the heart and liver, using echocardiography and abdominal ultrasonography, respectively. Liver ultrasonography performed 6 months prior to diagnosis did not show any focal lesions in the liver, except for Fontan-related liver cirrhosis with mild splenomegaly (12.5 cm). However, CT angiography to assess the Fontan tract unexpectedly revealed a 4 cm-sized hepatic mass in the right lobe (segment V), showing hyperenhancement in the arterial phase and washout in the portal venous phase (Fig. 2). To evaluate the mass further, the patient was referred to a hepatologist and underwent additional blood and imaging tests.
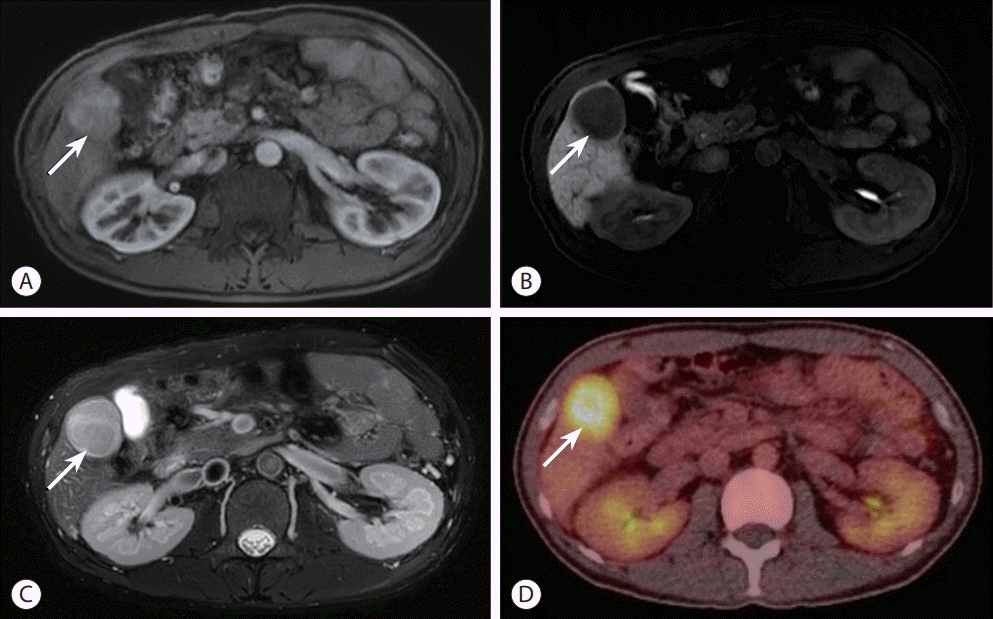
The initial blood test results at the time of diagnosis were as follows: white blood cell count, 6,350/µL; hemoglobin, 19.5 g/dL; platelet count, 145,000/µL; aspartate aminotransferase, 37 U/L; alanine aminotransferase, 33 U/L; total bilirubin, 1.5 mg/dL; total protein, 8.0 g/dL; serum albumin, 4.4 g/dL; and prothrombin time-international normalized ratio, 1.15. Hepatitis B surface antigen and hepatitis B core antibody tests were negative with anti-HBs antibody positive. The initial serum alpha-fetoprotein (AFP) level was 274.43 ng/mL, and protein induced by the absence of vitamin K or antagonist-II level was 52 mAU/mL. Liver function was preserved with Child-Turcotte-Pugh class A, and the Eastern Cooperative Oncology Group performance status was 0. The liver stiffness measured by transient elastography was 20.0 kPa. Gadoxetic acid-enhanced liver magnetic resonance imaging (MRI) also demonstrated a 4 cm-sized well-circumscribed mass with arterial phase hyperenhancement and hepatobiliary phase defect (Fig. 3A, B). It showed a high signal intensity on T2-weighted images (Fig. 3C) and diffusion restriction. On positron emission tomography-CT, the mass showed significant hypermetabolism, but there were no other abnormal hypermetabolic lesions, suggesting extrahepatic metastasis (Fig. 3D).
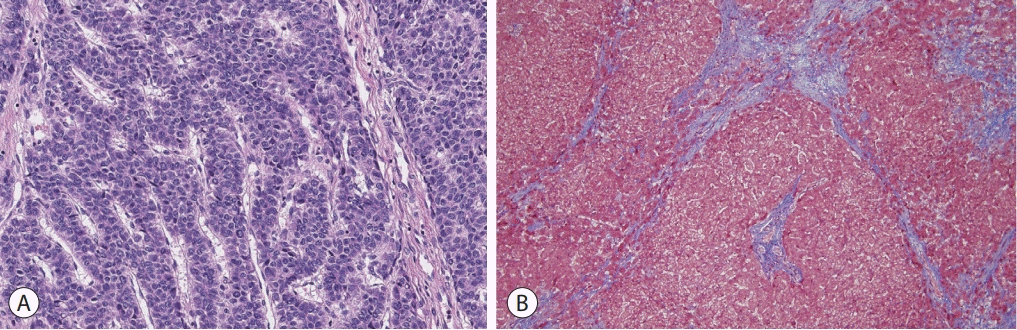
Based on the patient’s history of the Fontan operation, underlying liver cirrhosis, elevated AFP, and the results of various imaging studies, the hepatic mass was strongly suggestive of HCC. As there was no evidence of extrahepatic metastasis and the patient’s liver function was well preserved, laparoscopic non-anatomical S5 segmentectomy and cholecystectomy were performed. The resected specimen revealed a solid mass with a maximum diameter of 4.5 cm, and histological examination revealed a moderately differentiated HCC with a trabecular pattern (Fig. 4A). The background liver showed micronodular venocentric cirrhosis (Fig. 4B), consistent with cardiac cirrhosis. The patient was discharged without any complications after hepatic resection and was regularly followed-up. Serum AFP levels returned to the normal range after the operation, and there was no evidence of HCC recurrence up to 3 years after the initial treatment.
In patients who underwent the Fontan operation, the focus of care has shifted from early survival to long-term extracardiac complications as advancements in surgical techniques and postoperative management have led to a 20-year transplant-free survival rate of 83% among early survivors.6,7 The estimated global post-Fontan population is greater than 70,000, and is expected to double within the next decade.8 Due to the innate characteristics of Fontan circulation, liver damage from chronic hepatic congestion accumulates and can result in liver cirrhosis. Vascular shear stress and increased parenchymal stiffness may directly promote the activation of hepatic myofibroblasts (i.e., hepatic stellate cells).9,10 In addition, as cardiac output is usually lowered after the Fontan operation due to decreased preload, intermittent hypoxic and hypotensive insults may lead to centrilobular ischemia and further exacerbate liver cirrhosis.3
The most important risk factor for liver cirrhosis in postFontan patients is the duration after the operation.11 In one retrospective study, 86% of patients who underwent Fontan operation developed liver cirrhosis 20 years after the procedure.12 In another study, as more than 10 years had passed following the operation, the cumulative incidence rate of cirrhosis increased dramatically and nearly all of the surviving patients (97.9%) were confirmed to have liver cirrhosis 30 years after the procedure.5 Among 221 patients with established cirrhosis, seven (3.2%) were diagnosed with HCC during surveillance; however, no HCC was identified in 92 subjects without cirrhosis. Therefore, more frequent and thorough surveillance of hepatic complications should be considered in patients with a history of Fontan operation more than 10 years ago.
HCC is one of the most serious late complications in post-Fontan patients. Although the estimated annual risk of HCC (1.5-5.0%) in post-Fontan cirrhosis is comparable to that in cirrhosis of other etiologies,13 regular surveillance should be emphasized, considering the possibility of HCC occurrence at a very young age (before 20 years)14,15 and the relatively high mortality of HCC after Fontan operation.16-18 However, benign arterial-phase hyperenhancing nodules are frequently observed following Fontan operation,19 making the accurate diagnosis of HCC challenging because they might show washout on portal venous phase and/or delayed phase imaging.20,21 In addition, the limited utility of liver biopsy and MRI imaging due to coagulopathies and implanted cardiac devices in post-Fontan patients further complicates the detection of HCC.
Currently, there are no established guidelines for surveillance of HCC in patients after the Fontan procedure. It is generally accepted that patients who underwent the Fontan operation more than 10 years ago should receive annual abdominal ultrasonography and liver enzyme tests.3 If cirrhosis is confirmed, liver ultrasonography combined with serum AFP test should be performed every 6 months. Multiphase liver CT or MRI can be used when suspicious nodules are detected, unless there are any contraindications for MRI imaging, such as a pacemaker. However, in a retrospective study, 80% of patients diagnosed with HCC after Fontan operation showed no evidence of chronic liver disease on liver imaging performed within 2 years prior to the detection of HCC.22 The vicious cycle between reduced cardiac function and liver cirrhosis might be attributable to rapid tumor progression, since Fontan circulation failure is frequently observed in patients with established cirrhosis and decreased cardiac output further exacerbates fibrotic change.12 The low sensitivity and specificity of abdominal ultrasonography in post-Fontan patients also makes the early diagnosis of HCC challenging. Surface irregularity is a non-specific feature of FALD, as opposed to other chronic diseases,3 and it has been shown that liver nodules in post-Fontan patients can be easily missed owing to the low sensitivity of ultrasonography.23 Therefore, additional research is warranted to establish comprehensive guidelines for HCC surveillance in FALD patients.
In this report, we described a case of HCC diagnosed in a patient who had undergone Fontan operation 30 years ago. As the risk of FALD increases with the time elapsed since the operation, regular surveillance should be emphasized, especially in patients with liver cirrhosis. Considering the distinctive features of HCC in post-Fontan patients, specialized guidance for HCC surveillance in patients with FALD needs to be established.
Notes
Conflicts of Interest
Haeryoung Kim is currently Editor-in-Chief of J Liver Cancer. She was not involved in the review process of this article. Otherwise, the authors have no conflicts of interest to disclose.
Ethics Statement
This study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB No. 1812-158-998), and the requirement for informed consent was waived owing to the use of preexisting medical records.
REFERENCES
1. Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971; 26:240–248.
2. Gewillig M. The Fontan circulation. Heart. 2005; 91:839–846.
3. Gordon-Walker TT, Bove K, Veldtman G. Fontan-associated liver disease: a review. J Cardiol. 2019; 74:223–232.
4. Munsterman ID, Duijnhouwer AL, Kendall TJ, Bronkhorst CM, Ronot M, van Wettere M, et al. The clinical spectrum of Fontanassociated liver disease: results from a prospective multimodality screening cohort. Eur Heart J. 2019; 40:1057–1068.
5. Yoon JS, Lee DH, Cho EJ, Song MK, Choi YH, Kim GB, et al. Risk of liver cirrhosis and hepatocellular carcinoma after Fontan operation: a need for surveillance. Cancers (Basel). 2020; 12:1805.
6. Gersony WM. Fontan operation after 3 decades: what we have learned. Circulation. 2008; 117:13–15.
7. Khairy P, Fernandes SM, Mayer JE Jr, Triedman JK, Walsh EP, Lock JE, et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008; 117:85–92.
8. Emamaullee J, Zaidi AN, Schiano T, Kahn J, Valentino PL, Hofer RE, et al. Fontan-associated liver disease: screening, management, and transplant considerations. Circulation. 2020; 142:591–604.
9. Olsen AL, Bloomer SA, Chan EP, Gaça MD, Georges PC, Sackey B, et al. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol. 2011; 301:G110–G118.
10. Iwakiri Y, Shah V, Rockey DC. Vascular pathobiology in chronic liver disease and cirrhosis - current status and future directions. J Hepatol. 2014; 61:912–924.
11. Baek JS, Bae EJ, Ko JS, Kim GB, Kwon BS, Lee SY, et al. Late hepatic complications after Fontan operation; non-invasive markers of hepatic fibrosis and risk factors. Heart. 2010; 96:1750–1755.
12. Shimizu M, Miyamoto K, Nishihara Y, Izumi G, Sakai S, Inai K, et al. Risk factors and serological markers of liver cirrhosis after Fontan procedure. Heart Vessels. 2016; 31:1514–1521.
13. Asrani SK, Warnes CA, Kamath PS. Hepatocellular carcinoma after the Fontan procedure. N Engl J Med. 2013; 368:1756–1757.
14. Yamada K, Shinmoto H, Kawamura Y, Wakamatsu H, Kawauchi T, Soga S, et al. Transarterial embolization for pediatric hepatocellular carcinoma with cardiac cirrhosis. Pediatr Int. 2015; 57:766–770.
15. Kogiso T, Tokushige K. Fontan-associated liver disease and hepatocellular carcinoma in adults. Sci Rep. 2020; 10:21742.
16. Possner M, Gordon-Walker T, Egbe AC, Poterucha JT, Warnes CA, Connolly HM, et al. Hepatocellular carcinoma and the Fontan circulation: clinical presentation and outcomes. Int J Cardiol. 2021; 322:142–148.
17. Rodriguez De Santiago E, Téllez L, Guerrero A, Albillos A. Hepatocellular carcinoma after Fontan surgery: a systematic review. Hepatol Res. 2021; 51:116–134.
18. Egbe AC, Poterucha JT, Warnes CA, Connolly HM, Baskar S, Ginde S, et al. Hepatocellular carcinoma after Fontan operation: multicenter case series. Circulation. 2018; 138:746–748.
19. Asrani SK, Asrani NS, Freese DK, Phillips SD, Warnes CA, Heimbach J, et al. Congenital heart disease and the liver. Hepatology. 2012; 56:1160–1169.
20. Wells ML, Hough DM, Fidler JL, Kamath PS, Poterucha JT, Venkatesh SK. Benign nodules in post-Fontan livers can show imaging features considered diagnostic for hepatocellular carcinoma. Abdom Radiol (NY). 2017; 42:2623–2631.
21. Wells ML, Fenstad ER, Poterucha JT, Hough DM, Young PM, Araoz PA, et al. Imaging findings of congestive hepatopathy. Radiographics. 2016; 36:1024–1037.
22. Nandwana SB, Olaiya B, Cox K, Sahu A, Mittal P. abdominal imaging surveillance in adult patients after fontan procedure: risk of chronic liver disease and hepatocellular carcinoma. Curr Probl Diagn Radiol. 2018; 47:19–22.
23. Téllez L, Rodríguez de Santiago E, Minguez B, Payance A, Clemente A, Baiges A, et al. Prevalence, features and predictive factors of liver nodules in Fontan surgery patients: The VALDIG Fonliver prospective cohort. J Hepatol. 2020; 72:702–710.
Figure 1.
Effect of Fontan operation on the development of Fontan-associated liver disease. In Fontan circulation, the systemic veins and pulmonary arteries are directly connected. Elevated central venous pressure induced by passive filling of the pulmonary circulation without the support of the right ventricle results in chronic hepatic congestion. In addition, low cardiac output due to limited preload reduces portal blood flow, which contributes to ischemic insults. Consequently, accumulated liver damage can lead to liver cirrhosis and even hepatocellular carcinoma.
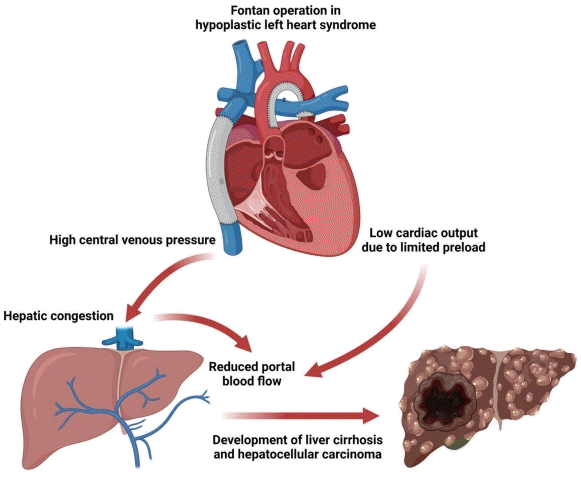
Figure 2.
Computed tomography image showing a 4 cm-sized hepatic mass in segment V (arrows). The mass is seen as hyperenhancement in the arterial phase (A) and washout in the portal venous phase (B).
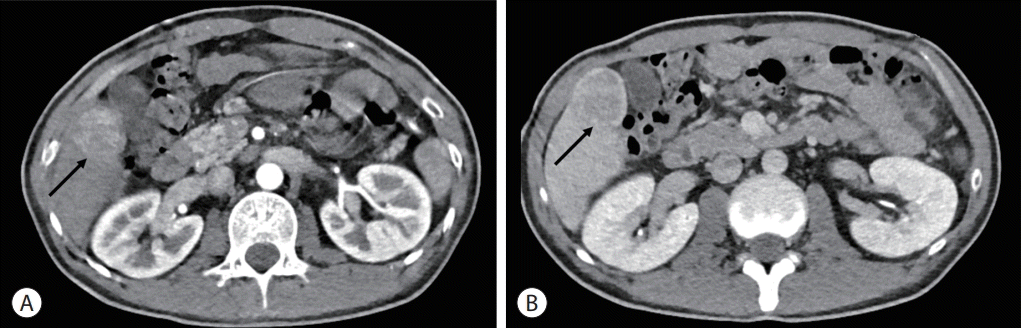
Figure 3.
Contrast-enhanced liver magnetic resonance imaging showing a well-circumscribed mass (arrow) with arterial phase hyperenhancement (A) and hepatobiliary phase defect (arrow) (B). In a T2-weighted image, it shows a high signal intensity (arrow) (C). The hepatic mass exhibited significant hypermetabolism in the positron emission tomography-computed tomography image (arrow) (D).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download