Abstract
Purpose
Patients undergoing pancreatoduodenectomy are a high-risk group that requires psychosocial support. This study retrospectively reviewed the prevalence of psychological symptoms in patients undergoing pancreatoduodenectomy for periampullary neoplasm and the psychosocial referral rate after implementing full screening and triage algorithm for administering a distress management protocol based on the integrated supportive care system established in 2010.
Materials and Methods
From September 2010 to December 2018, insomnia, anxiety, and depression were screened on the first day of admission (T1) and on the 10th postoperative day (T2). Patients with clinical levels of distress were referred to a mental health clinic for appropriate aftercare.
Results
The adherence rate to routine screening was 82.7% (364/440). Among the 364 patients, the prevalence of insomnia, anxiety, and depression increased from 22.0% (T1) to 32.6% (T2, p=0.001), 29.1% to 33.6% (p=0.256), and 18.4% to 27.6% (p=0.001), respectively. Less than 45% of those with psychological symptoms expressed their needs for psychological supportive care. Among those with psychological symptoms at T2, clinical insomnia, anxiety, and depression were detected via in-depth evaluations among 77.2%, 38.1%, and 82.5% of patients, respectively. Patients who had two or more symptoms at T2 had a longer postoperative hospital stay, as compared to those with one or no symptoms (a median of 20.5 days vs. 18.0 days, p=0.006). Psychiatric consultation rate was 72.8% among patients with clinical psychological symptoms, and 74% of the consulted patients completed psychiatric intervention before discharge.
Patients with newly diagnosed/recurred cancer or those receiving active cancer treatment have several physical/psychological symptoms [1–3]. Given the impact of poorly managed distress on quality of life, targeted supportive care should be provided to patients with a higher risk of symptom burden, especially during the first few months of a diagnosis when the odds of moderate-to-severe psychological symptoms are high [4]. However, despite its importance for distress screening and management, patients’ distress continues to be under-recognized. Distress screening is more effective when it is linked with mandatory intervention or referral [5].
Periampullary neoplasm is characterized by its grave prognosis and the need for a pancreatoduodenectomy, which has a high morbidity rate; both these aspects result in higher psychological stress among patients compared to other cancers. The prevalence of anxiety or depression is higher for those with pancreatic cancer than other cancer sites [1]; after six months of undergoing a pancreatectomy, 17%–33% and 7%–21% of disease-free survivors had anxiety or depression [6,7]. Additionally, 52% of the patients with periampullary cancer reported that they had an unmet need: psychological supportive care [8]. However, despite the high interest in pancreatoduodenectomy (due to its potential impact on the quality of life), few studies have focused on psychological distress that might occur after the operation [9]. While a recent study from the Ontario Cancer Registry reveals that 51.4% and 39.5% of patients report moderate-to-severe anxiety or depression during the first 12 months after pancreatoduodenectomy [10], the prevalence of psychological distress and need for supportive care that patients with periampullary neoplasm experience immediately after a pancreatoduodenectomy have not yet been sufficiently studied.
In South Korea, only 1.3% of patients had psychiatric consultations in 2006, reflecting the under-recognized burden of psychological distress that cancer patients experience [11]. Thus, distress management guidelines for Korean cancer patients were developed in 2009 [11]. Accordingly, an integrated supportive care system was established in 2010 at the Center for Liver and Pancreatobiliary Cancer, National Cancer Center, Korea, to provide multidisciplinary management for patients undergoing a pancreatoduodenectomy experience. A distress management protocol comprising a routine screening and referral system for psychological distress (e.g., insomnia, anxiety, and depression) was included in the integrated supportive care system. This study retrospectively investigates patients with periampullary neoplasm undergoing pancreatoduodenectomy, including the prevalence of psychological symptoms, related factors, and the responsiveness of surgeons to clinically significant symptoms after implementing the distress management protocol.
As part of our institute’s “critical pathways”, we organized a multidisciplinary team of experts, including surgeons, dietitians, endocrinologists, anesthesiologists, physiatrists, psychiatrists, and psychologists, all having substantial experience in supportive care of cancer patients. Each specialization provided personalized care e.g., nutritional support, diabetes control, pain management, preoperative and postoperative rehabilitation, and psychiatric intervention to patients before and after pancreatectomy according to its own management protocols (S1 Fig.). Surgeons were responsible for the integrated management of the patients and made referrals to relevant specialists upon problem detection.
For psychological distress management, we developed a distress management protocol for patients undergoing pancreatoduodenectomy based on the Korean distress management guidelines [11]. This protocol included a routine screening and systematic psychiatric referral system for psychological symptoms based on the two-tiered and triage model. The patients’ psychological symptoms were categorized into three domains (insomnia, anxiety, and depression), based on commonly experienced psychiatric symptoms in cancer patients [12]. Patients were routinely screened for symptoms at the time of admission (T1) and on the tenth postoperative day (T2). For those who had psychological symptoms at T2, an in-depth evaluation of their symptoms was performed on the same day. The screening and in-depth questionnaire were administered by a registered nurse, who assisted the patients in reading, understanding, and completing the questionnaire. Then, a clinical psychologist interpreted the test score, decided the pathway for care according to the algorithm, and recommended aftercare to the surgeon. The clinical psychologist recommended emotional support and the provision of educational medical information by the primary medical staff to patients with normal to mild distress levels. Referral for psychiatric intervention was provided to patients with mild-to-severe levels of distress. For patients who did not complete the in-depth evaluation, whether to refer to mental health services was decided based on the results of the T2 screening. Surgeons referred those with clinically significant symptoms to psychiatric consultation for receiving relevant management. The psychiatrists and/or psychologists visited the patients for examination and consultation. Patients who were referred to the mental health clinic received pharmacological and/or non-pharmacological intervention, such as psychotherapy and psychoeducation. The median hospital stay of the study population was 25 days (range, 12 to 123 days); hence, all the eligible patients were in admission when T2 screening was performed and the in-depth questionnaire was administered. The surgeons were responsible for supervising the registered nurse and making referrals to the psychiatrists and psychologists. A screening and consultation request system was implemented on the electronic medical records (EMR) system to enable communication among the experts. Screening results at T1 mainly served as the baseline, but occasionally, the patient was referred to a psychiatrist when it was determined that psychiatric treatment was required.
In this retrospective study, de-identified archival data was analyzed, while the serial data of patients who underwent a pancreatoduodenectomy for a periampullary neoplasm (from September 2010 to December 2018) were retrospectively reviewed. Patients whose operations were canceled, or who did not otherwise undergo planned pancreatoduodenectomy, were excluded from analysis. Among the 440 patients admitted for the procedure during the study period, 364 patients who completed screening of psychological symptoms at T1 and underwent pancreatoduodenectomy were included in the analysis.
Screening was performed at T1 and T2 by measuring psychological symptoms with the Korean version of the self-reported National Cancer Center Psychological Symptom Inventory (NCC-PSI) [12]. The NCC-PSI consists of six items on an eleven-point visual analogue scale that measures the severity and impact of insomnia, anxiety, or depression over the past week. One item enquires about the “need for help from mental health experts” for each symptom [12]. The cutoff score in the NCC-PSI for clinically significant insomnia, anxiety, and depression was five, four, and four points, respectively [12].
In-depth questionnaires were administered to patients who had NCC-PSI scores higher than the cutoff score at T2 to triage patients who needed priority psychiatric intervention. The severity of insomnia was measured using the Korean version of the Insomnia Severity Index (ISI) [13,14]. Each of the seven items was rated on a five-point Likert scale (0, not at all; 4, very severe), and the range of total scores for insomnia was interpreted as not being clinically significant (0–7 points), mild (8–14 points), moderate (15–21 points), or severe (22–28 points) [14]. Meanwhile, the severity of state anxiety was measured using the Korean version of the self-reported State-Trait Anxiety Inventory (STAI-X) [15,16]. Each of the 20 items was rated on a four-point Likert scale (1, almost never; 4, almost always), and the results for the patient’s anxious state were classified as insignificant (20–51 points), mild (52–56 points), moderate (57–61 points), or severe (62–80 points) [16]. Finally, the level of depression over the past week was measured using the Korean version of the self-reported Beck Depression Inventory (BDI) [17,18]. Each of the 21 items was rated via four possible responses (0–3), and the severity of depression was categorized as insignificant (0–9 points), mild (10–15 points), moderate (16–23 points), or severe (24–63 points) [18]. By utilizing the cutoff score suggested by the scale, questionnaire results rated as mild-to-severe were considered clinically significant.
The demographic and clinical data from the EMR were retrospectively collected, including the following: age; sex; the status of marriage, employment, and education; smoking and alcohol intake; past psychiatric history; medication; family history of cancer; insight into one’s disease; cancer type; and the European Cooperative Oncology Group (ECOG) performance scale at the time of hospitalization. In addition, the following data were collected: patients’ nutritional status (via a patient-generated subjective global assessment [PG-SGA]) at T1, any complications that developed within ten days after the operation, and the highest pain score on the 10th postoperative day (T2) as well as the postoperative hospital stay.
Continuous variables were expressed as median and interquartile range (IQR) and compared using Mann-Whitney U tests. The serial NCC-PSI scores at T1 and T2 were compared using Wilcoxon signed-rank tests. The categorical variables were compared using the chi-square or Fisher exact test, and the correlation was tested via Spearman’s correlation test. All the statistical analyses, which were performed using STATA ver. 16.1 (StataCorp LLC, College Station, TX), were two-sided, and the statistical significance was defined as p < 0.05.
The adherence rate for routine screening psychological symptoms at the time of admission was 82.7% (364/440) for all the patients who underwent a pancreatoduodenectomy during the study period. Of the 364 patients, 91.8% (n=334) completed their screening at T2. Response rates for the in-depth questionnaires were 80.7% (88/109, ISI), 75.0% (84/112, STAI-X), and 68.5% (63/92, BDI), respectively. Although all the questionnaires were administered one-on-one, patients tended to refuse to answer it due to their poor general condition or to drop out when the questionnaire was lengthy (e.g., STAI-X or BDI). In addition, psychiatric referrals were occasionally omitted because the referral process was not electronically automated and had to be handled by the surgeons (S2 Fig.).
The median annual number of patients was 45 (IQR, 37 to 48), and their demographics are presented in Table 1. The patients with distal common bile duct cancer included a higher proportion of men (80.2% [n=65] vs. 63.6% [n=96, pancreatic head], 55.4% [n=46, ampulla of Vater], 61.2% [n=30, other types]; p=0.007), and the proportion of patients with an ECOG score that was higher than or equal to two was higher for those with pancreatic head cancer (11.3% [n=17] vs. 2.5% [n=2, distal common bile duct], 7.2% [n=6, ampulla of Vater], 2.0% [n=1, other types]; p=0.039). Other demographic factors were comparable across the primary sites of disease.
The prevalence of insomnia, anxiety, or depression at T1 was 22.0% (n=80), 29.1% (n=106), and 18.4% (n=67), respectively, and after the operation, these percentages increased by 10.6%, 4.5%, and 9.2% (Fig. 1). At T1 and T2, 37.9% (138/364 [T1]) and 46.4% (155/334 [T2]) of patients had psychological symptoms in at least one of the three domains. However, less than 15% and 20% of all patients at T1 and T2, respectively, expressed their “need for help from mental health experts” for their psychological symptoms through the items on the screening questionnaire (NCC-PSI). Among those with scores higher than the cutoff, less than 45% expressed the need for psychological assistance, and this frequency was even lower in patients with depression, compared to those with insomnia or anxiety. The presence of psychological symptoms at T1 and T2 showed significant correlation (rs=0.173, p=0.002 [insomnia]; rs=0.352, p < 0.001 [anxiety]; rs=0.353, p < 0.001 [depression]). Each domain illustrated significant cross-correlation (insomnia and anxiety, p < 0.001; insomnia and depression, p < 0.001; anxiety and depression, p < 0.001), especially the patients with depression who had concurrent anxiety, 92.5% (n=62) at T1 and 84.8% (n=78) at T2.
For the screening test at T2, the patients who exceeded the cutoff score across the three domains were evaluated in-depth for the relevant domain. The ISI was completed for 80.7% (88/109) of eligible patients, and of these, 77.2% (n=68) had a mild-to-severe level of insomnia. As shown in Fig. 2A, 28.4% of patients who did not have clinically significant insomnia at T1 developed symptoms after the operation. Meanwhile, the STAI-X was completed for 75.0% (84/112) of eligible patients, and of these, 38.1% (n=32) had either a mild-to-severe level of state anxiety. Among the patients with clinically significant anxiety at T1, 59.0% (n=59) remained symptomatic after the operation (Fig. 2B). The proportion of patients who had anxiety at T1 was higher than those who did not (85.7% [n=12] vs. 14.3% [n=2], p=0.011) among 14 patients with moderate-to-severe anxiety. Finally, BDI was completed for 68.5% (63/92) of eligible patients, of whom 82.5% (n=52) had either a mild or higher level of depression. Sixty percent of the patients who experienced clinically significant depressive symptoms at T1 remained symptomatic after the operation (Fig. 2C).
Related to the clinical level of psychological symptoms at T1 and T2, we analyzed pre- and postoperative factors listed in the “Demographic and clinical variables” section. At T1, insomnia was related to having a poor nutritional status, no previous history of alcohol intake, and a past history of depression/insomnia. Additionally, a history of depression was related to having anxiety/depression at T1 (Table 2).
The postoperative hospital stay was significantly longer for patients who had clinically relevant anxiety (median, 21 days [IQR 16–29] vs. 18 days [IQR 14–23]; p=0.001) or depression (median, 21 days [IQR 16–29] vs. 18 days [IQR 15–24]; p=0.007) at T2. Except for elevated serum C-reactive protein level in patients with clinically relevant anxiety (median, 8.5 [IQR 2.9, 13.4] vs. 4.7 [IQR 1.3, 10.6]; p=0.028), postoperative complications [any complications, Clavien-Dindo classification grade ≥ III, postoperative pancreatic fistula grade ≥ B, delayed gastric emptying grade ≥ B, postoperative hemorrhage requiring transfusion, superficial surgical site infection only], serum hemoglobin, serum C-reactive protein, and numeric rating scale of pain did not have statistical significance in relation to clinically relevant insomnia, anxiety or depression at T2.
Meanwhile, patients who had two or more symptoms at T2 had a longer postoperative hospital stay, as compared to those with one or no symptoms (a median of 20.5 [IQR, 16.0 to 29.0] vs. 18.0 [IQR, 14.3 to 24.0], p=0.006). Although, according to the number of symptoms, notably, the following factors were not significantly different: age (a median of 67.0 [IQR, 58.0 to 72.0] vs. 67.0 [IQR, 59.0 to 72.0], p=0.911) and complication grade (Clavien-Dindo classification ≥ grade 3, 45.1% [n=23] vs. 54.9% [n=28], p=0.344).
A multivariate analysis of the effect of psychological symptoms and postoperative complications on postoperative hospital stay revealed that clinically relevant anxiety at T2 (odds ratio, 4.930; 95% confidence interval [CI], 1.300 to 8.559; p=0.008), postoperative pancreatic fistula grade ≥ B (odds ratio, 13.115; 95% CI, 9.443 to 16.796; p < 0.001), and delayed gastric emptying grade ≥ B (odds ratio, 12.314; 95% CI, 8.036 to 16.591; p < 0.001) were significant factors related with postoperative hospital stay.
After the psychological symptoms screening at T2, 31.1% (n=104) of all patients had a psychiatric consultation, while 52.9% (n=82) who had psychological symptoms at T2 were provided this service as well. Overall, Among the 92 patients who had a mild-to-severe level of in-depth questionnaire scores, 72.8% (n=67) underwent psychiatric consultation.
Among the 104 patients who had in-patient referrals, 82.7% (n=86) and 57.7% (n=60) were treated with psychotherapy or medication, respectively. The median number of psychotherapy sessions was one (IQR, 1 to 2), while medication was prescribed for a median of 13 days (IQR, 6 to 29). The top three frequent in-hospital medications prescribed were zolpidem (28 patients; IQR, 3 to 10 days), quetiapine (19 patients; IQR, 12 to 32 days), and lorazepam (18 patients; IQR, 7 to 20 days). Twenty-seven patients required follow-ups at the out-patient clinic due to persistent symptoms; 100% (n=27) and 59.3% (n=16) received psychotherapy and medication, respectively. After discharge, the median number of psychotherapy sessions that were performed at the out-patient clinic was one (IQR, 1 to 3), while medication was prescribed for a median of 28 days (IQR, 22 to 43).
The study results showed that about one-third of the respondents experienced insomnia, anxiety, or depression after pancreatectomy, which was comparable to the out-patient clinical data that was collected one month after the pancreatoduodenectomy [10]. However, the prevalence was higher than the prevalence experienced by patients 6 months after the operation [6], which is concordant with the decreasing level of psychological symptoms after surgery [10]. The prevalence of psychological symptoms observed in this study increased from 4.5% to 10.6% across the three symptoms after the pancreatoduodenectomy, which is unlike breast cancer patients, whose anxiety significantly decreased after their operation [19]. About 45% of the patients had psychological symptom comorbidly at T2 and those who had two or more symptoms at T2 had a longer postoperative hospital stay, as compared to those with one or no symptoms. These findings imply that psychological supportive care should be provided at the onset of surgical treatment when the burden of psychological symptoms is the highest.
The risk factors of being younger or female has been reported as being associated with cancer patients’ symptom burden [4], but it was not found to be significant in this study. Although psychological symptoms before surgery did not reveal direct correlations with postoperative outcomes, a considerable proportion of patients who had anxiety (59.0%) or depression (60.3%) at T1 remained symptomatic until T2, especially those with anxiety at T1, as they were more likely to develop moderate-to-severe anxiety by T2. Meanwhile, 82.5% of patients with depression at T2 had a mild-to-severe level of symptoms. Moreover, patients with anxiety or depression at T2 experienced a longer postoperative hospital stay. Therefore, patients screened as having either anxiety or depression perioperatively should be the main target of receiving improved psychological supportive care. Psychological symptom screening before surgery would help identify those who are at higher risk of developing symptoms after surgery.
This study revealed that only 44.6% and 25.0% of the patients who experienced clinical levels of anxiety or depression after operation expressed their need for help from mental health experts. This could be because of the patient’s lack of awareness of their own symptoms or misunderstanding or prejudices about mental health services. Therefore, it is important for the clinician to recognize the patient’s need for help, but above all, it is important to objectively evaluate psychological distress. The patient’s needs can also be considered along with the scale scores for determining psychiatric referrals. Given the absence of routine screening in the majority of surgical wards, it is important to improve surgeons’ understanding about the prevalence of patients’ psychological symptoms before and after a pancreatoduodenectomy to address the latter’s psychological needs. This is also supported by other research; in Australia, for example, only 15% of patients with periampullary cancer voluntarily sought psychological supportive care [8]. Similarly, in Korea, psychological distress, or psychiatric disorders, in cancer patients remain an under-recognized phenomenon [20]. Moreover, psychiatric referrals are still the only way for hospitalized patients to receive psychological interventions as part of their cancer treatment [11].
Importantly, the rate of psychiatric consultations was less than 2% in the authors’ institution before the introduction of a routine screening for psychological symptoms with a systematic psychiatric consultation protocol [11]. After the protocol was implemented, the adherence rate for initial psychological symptom screening was 82.7%, and 52.9% of patients with psychological symptoms and 72.8% of patients with a mild-to-severe level psychological symptoms, specifically, were referred to psychiatrists for consultations. This is as high as in the United States, where since 2015, screening for psychosocial distress and relevant referrals have been made mandatory for cancer center accreditation by the American College of Surgeons Commission on Cancer [21].
Meanwhile, patient-reported outcomes (PROs) are one of the important outcome measures in terms of health care’s quality, efficiency, and safety [22]. Recently suggested sets of PROs [23] or value-based, patient-centered outcomes [24] for pancreatic cancer have included a list of psychological/emotional symptoms, which should be validated and incorporated in future outcome-based research. Although it is difficult to prove a direct correlation between PROs and quantitative treatment responses, adopting PROs in clinical research may foster the provision of patient-centered health care by considering the patients’ perspectives in both clinical decision-making as well as health policy formulation.
This study has several limitations. First, the study was a retrospective analysis. Therefore, reasons for not adhering to the newly implemented in-patient protocol including psychological screening and psychiatric consultation were not identified from both the patients’ and surgeons’ perspective. A possible underestimation of the rate of psychological symptoms may have occurred, as there were cases of patients not responding at all or refusing to participate in in-depth evaluation due to their poor condition. Psychiatric consultation rates might have been influenced by the surgeons’ preferences given the long timeframe included in analysis. Additionally, since we carried out the research at a single site, its results are limited in generalizability. Although this study offers limited evidence to support the feasibility of a multicenter study, the observed adherence rate to the newly implemented in-patient protocol was 82.7%. This rate might serve as a baseline when planning future, larger scale studies. Third, having no control group, it was difficult to evaluate whether implementing a routine psychological screening using a systematic psychiatric consultation protocol improved the treatment outcomes for patients. Although more patients received psychiatric consultations and treatments than was the case in the past, it is difficult to conclude that these interventions enhanced early recovery postoperation. Fourth, the psychological symptoms were only evaluated for in-patients; therefore, long-term psychological outcomes could not be identified. Moreover, the improvement of psychological symptoms after psychological intervention could not be assessed quantitatively because of minimized out-patient visits due to the social taboo of visiting psychiatric clinics and human resource limitations in administering questionnaires at surgical out-patient clinics. However, 74.0% of the patients who had an in-hospital referral did not require out-patient clinical follow-up after being discharged, suggesting that their symptoms had been relieved before discharge. Finally, this study did not evaluate the protocol’s cost-effectiveness. Therefore, there is limited evidence to suggest that the protocol implemented in this study should be adopted in other hospitals or institutions.
In summary, more or less than 40% of the patients who had periampullary neoplasm experienced psychological symptoms before and after their pancreatoduodenectomy, respectively. After implementing a routine screening of psychological symptoms with a systematic psychiatric consultation protocol, surgeons’ responsiveness to patients’ psychological symptoms increased compared with the rate gleaned from available historical data. Seventy-three percent of the patients who experienced a mild-to-severe level of psychological symptoms received psychiatric consultations after the routine screening of psychological symptoms as part of the integrated supportive care protocol, while psychological management was completed during the admission of 74% of the patients. Future studies should evaluate both short-term and long-term trends associated with the epidemiology of psychological symptoms after pancreatoduodenectomy. Additionally, the feasibility and cost-effectiveness of routine psychological supportive care must be assessed on a larger scale using a control group to direct the future reform of daily surgical practice.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethics Statement
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the human research committee of National Cancer Center, with exemption of written informed consent because of the retrospective study design (IRB No. NCC2019-0223).
References
1. Clark KL, Loscalzo M, Trask PC, Zabora J, Philip EJ. Psychological distress in patients with pancreatic cancer: an understudied group. Psychooncology. 2010; 19:1313–20.
2. Linden W, Vodermaier A, Mackenzie R, Greig D. Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J Affect Disord. 2012; 141:343–51.
3. Reilly CM, Bruner DW, Mitchell SA, Minasian LM, Basch E, Dueck AC, et al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer. 2013; 21:1525–50.
4. Bubis LD, Davis L, Mahar A, Barbera L, Li Q, Moody L, et al. Symptom burden in the first year after cancer diagnosis: an analysis of patient-reported outcomes. J Clin Oncol. 2018; 36:1103–11.
5. Mitchell AJ. Screening for cancer-related distress: when is implementation successful and when is it unsuccessful? Acta Oncol. 2013; 52:216–24.
6. Cloyd JM, Tran Cao HS, Petzel MQ, Denbo JW, Parker NH, Nogueras-Gonzalez GM, et al. Impact of pancreatectomy on long-term patient-reported symptoms and quality of life in recurrence-free survivors of pancreatic and periampullary neoplasms. J Surg Oncol. 2017; 115:144–50.
7. Petzel MQ, Parker NH, Valentine AD, Simard S, Nogueras-Gonzalez GM, Lee JE, et al. Fear of cancer recurrence after curative pancreatectomy: a cross-sectional study in survivors of pancreatic and periampullary tumors. Ann Surg Oncol. 2012; 19:4078–84.
8. Beesley VL, Janda M, Goldstein D, Gooden H, Merrett ND, O’Connell DL, et al. A tsunami of unmet needs: pancreatic and ampullary cancer patients’ supportive care needs and use of community and allied health services. Psychooncology. 2016; 25:150–7.
9. Lounis L, Aurran-Schleinitz T, Turrini O, Delpero JR, Brejard V. Psychological outcomes and quality of life in relation to pancreatectomy: a systematic review. Pancreas. 2019; 48:471–9.
10. _Tung S, Davis LE, Hallet J, Mavros MN, Mahar AL, Bubis LD, et al. Population-level symptom assessment following pancreaticoduodenectomy for adenocarcinoma. JAMA Surg. 2019; 154:e193348.
11. Yu ES, Shim EJ, Kim HK, Hahm BJ, Park JH, Kim JH. Development of guidelines for distress management in Korean cancer patients. Psychooncology. 2012; 21:541–9.
12. Shim EJ, Hahm BJ, Yu ES, Kim HK, Cho SJ, Chang SM, et al. Development and validation of the National Cancer Center Psychological Symptom Inventory. Psychooncology. 2017; 26:1036–43.
13. Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001; 2:297–307.
14. Cho YW, Song ML, Morin CM. Validation of a Korean version of the insomnia severity index. J Clin Neurol. 2014; 10:210–5.
15. Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press;1970.
16. Kim JT. A study of the relationship between trait anxiety and social tendency. J Human Underst Couns. 1978; 1:16–26.
17. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961; 4:561–71.
18. Lee YH, Song JY. A study of the reliability and the validity of the BDI, SDS, and MMPI-D scales. Korean J Clin Psychol. 1991; 10:98–113.
19. Kim J, Cho J, Lee SK, Choi EK, Kim IR, Lee JE, et al. Surgical impact on anxiety of patients with breast cancer: 12-month follow-up prospective longitudinal study. Ann Surg Treat Res. 2020; 98:215–23.
20. Lee HJ, Lee KM, Jung D, Shim EJ, Hahm BJ, Kim JH. Psycho-oncology in Korea: past, present and future. Biopsychosoc Med. 2017; 11:12.
21. Zebrack B, Kayser K, Sundstrom L, Savas SA, Henrickson C, Acquati C, et al. Psychosocial distress screening implementation in cancer care: an analysis of adherence, responsiveness, and acceptability. J Clin Oncol. 2015; 33:1165–70.
22. Prodinger B, Taylor P. Improving quality of care through patient-reported outcome measures (PROMs): expert interviews using the NHS PROMs Programme and the Swedish quality registers for knee and hip arthroplasty as examples. BMC Health Serv Res. 2018; 18:87.
23. van Rijssen LB, Gerritsen A, Henselmans I, Sprangers MA, Jacobs M, Bassi C, et al. Core Set of Patient-reported Outcomes in Pancreatic Cancer (COPRAC): an international delphi study among patients and health care providers. Ann Surg. 2019; 270:158–64.
24. Cherkaoui Z, Gonzalez C, Wakabayashi T, Delattre B, Leost E, Serra S, et al. A standard set of value-based patient-centered outcomes for pancreatic carcinoma: an international delphi survey. Ann Surg Oncol. 2021; 28:1069–78.
Fig. 1
Screening results on the first day of admission (T1) and the tenth postoperative day (T2) using National Cancer Center Psychological Symptom Inventory.
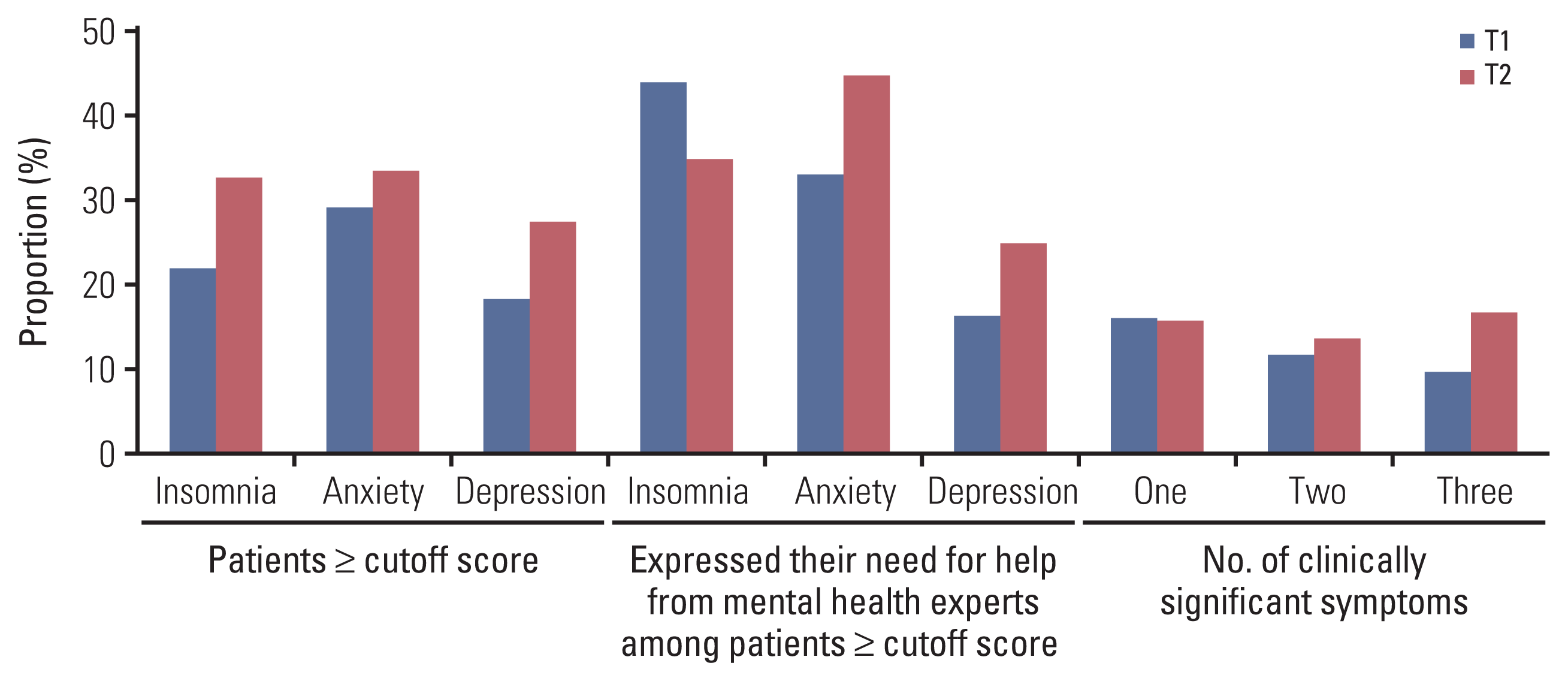
Fig. 2
Serial prevalence and severity of psychological symptoms at T1 and T2, and with in-depth questionnaires for symptomatic patients at T2: insomnia (A), anxiety (B), and depression (C). ISI, Insomnia Severity Index; STAI-X, Korean version of the self-reported State-Trait Anxiety Inventory.
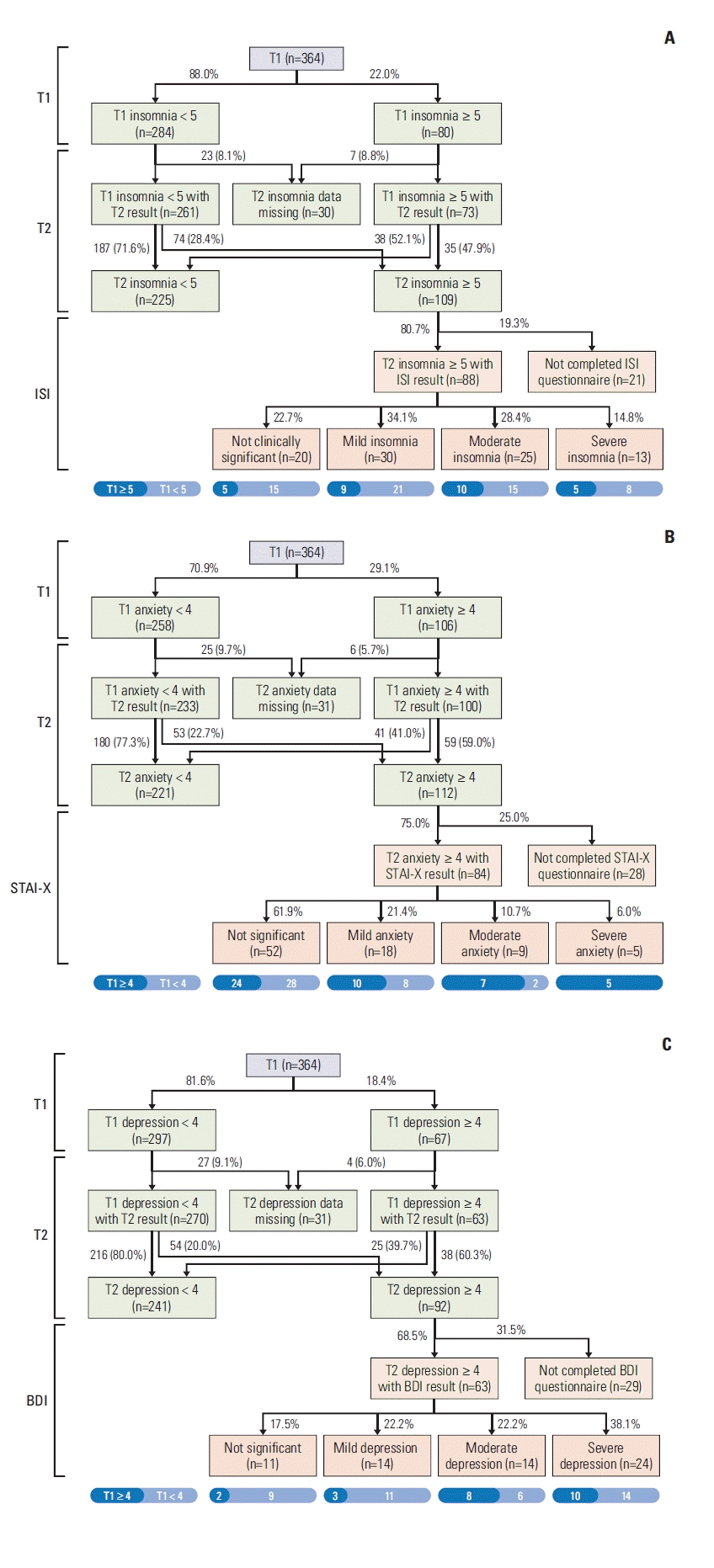
Table 1
Patients’ characteristics
| Variable | Value (n=364) |
|---|---|
| Age (yr) | 67 (59–72) |
| Male sex | 237 (65.1) |
| Spouse: yes, currently | 285 (81.0) |
| Employed: yes, currently | 168 (46.2) |
| Education | |
| Primary or less | 106 (29.1) |
| Secondary | 188 (51.6) |
| University or higher | 65 (17.9) |
| Missing | 5 (1.4) |
| Smoking | |
| Yes, any point of time | 189 (51.9) |
| Amount (pack-year) insomnia, | 31.0 (20.8–48.0) |
| Alcohol | |
| Yes, any point of time | 188 (51.6) |
| Amount (g) | 12.3 (4.1–40.3) |
| Past psychiatric history | |
| Insomnia | 13 (3.6) |
| Depression | 8 (2.2) |
| Malignancy: yes | 342 (94.0) |
| Primary diseases | |
| Pancreatic head cancer | 151 (41.5) |
| Distal common bile duct cancer | 81 (22.3) |
| Ampulla of Vater cancer | 83 (22.8) |
| Others | 49 (13.5) |
| Insight of disease | 342 (94.0)a) |
| European cooperative oncology group performance scale | |
| 0 | 237 (65.1) |
| 1 | 101 (27.7) |
| 2, 3, 4 | 26 (7.1) |
| Postoperative complication | 137/261 (52.5) |
| Clavien-Dindo classification, grade ≥ III | 53/261 (20.3) |
| Pancreatic fistula, grade ≥ B | 32/261 (12.3) |
| Delayed gastric emptying, grade ≥ B | 23/261 (8.8) |
| Hemorrhage requiring transfusion | 7/261 (2.7) |
| Postoperative in-hospital mortality | 1 (0.3) |
| Postoperative hospital stay (days) | 19 (15–27) |
| Serum hemoglobin at T2 (g/dL) | 10.1 (9.1–11.1) |
| Serum C-reactive protein at T2 | 6.2 (1.6–12.4) |
| Numeric rating scale of pain at T2 | 3 (1–5) |
Table 2
Factors associated with NCC-PSI scores over the cutoff values on baseline assessment (T1)




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download