1. Gregory RK, Powles TJ, Chang JC, Ashley S. A randomised trial of six versus twelve courses of chemotherapy in metastatic carcinoma of the breast. Eur J Cancer. 1997; 33:2194–7.
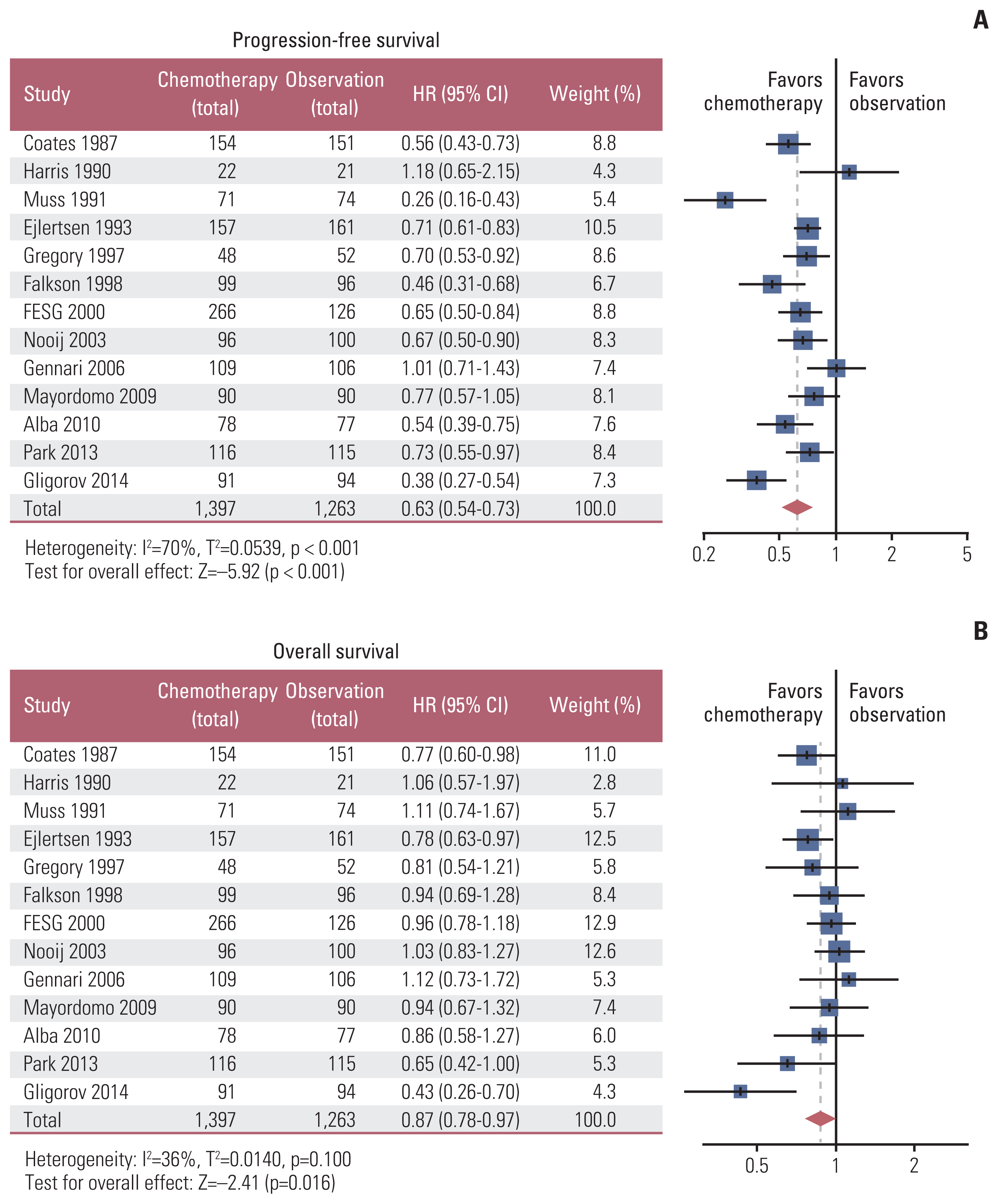
2. Muss HB, Case LD, Richards F 2nd, White DR, Cooper MR, Cruz JM, et al. Interrupted versus continuous chemotherapy in patients with metastatic breast cancer. The Piedmont Oncology Association. N Engl J Med. 1991; 325:1342–8.
3. Park YH, Jung KH, Im SA, Sohn JH, Ro J, Ahn JH, et al. Phase III, multicenter, randomized trial of maintenance chemotherapy versus observation in patients with metastatic breast cancer after achieving disease control with six cycles of gemcitabine plus paclitaxel as first-line chemotherapy: KCSG-BR07-02. J Clin Oncol. 2013; 31:1732–9.
4. Ejlertsen B, Pfeiffer P, Pedersen D, Mouridsen HT, Rose C, Overgaard M, et al. Decreased efficacy of cyclophosphamide, epirubicin and 5-fluorouracil in metastatic breast cancer when reducing treatment duration from 18 to 6 months. Eur J Cancer. 1993; 29A:527–31.
5. Tredan O, Follana P, Moullet I, Cropet C, Trager-Maury S, Dauba J, et al. A phase III trial of exemestane plus bevacizumab maintenance therapy in patients with metastatic breast cancer after first-line taxane and bevacizumab: a GINECO group study. Ann Oncol. 2016; 27:1020–9.
6. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009; 151:W65–94.
7. Higgins JP, Green S. Cochrane handbook for systematic reviews of iterventions, version 5.1. 0. Chichester: John Wiley & Sons Ltd;2011.
8. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
9. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7:177–88.
10. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–60.
11. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008; 336:924–6.
12. Westreich D, Lessler J, Funk MJ. Propensity score estimation: neural networks, support vector machines, decision trees (CART), and meta-classifiers as alternatives to logistic regression. J Clin Epidemiol. 2010; 63:826–33.
13. Alba E, Ruiz-Borrego M, Margeli M, Rodriguez-Lescure A, Sanchez-Rovira P, Ruiz A, et al. Maintenance treatment with pegylated liposomal doxorubicin versus observation following induction chemotherapy for metastatic breast cancer: GEICAM 2001-01 study. Breast Cancer Res Treat. 2010; 122:169–76.
14. Coates A, Gebski V, Bishop JF, Jeal PN, Woods RL, Snyder R, et al. Improving the quality of life during chemotherapy for advanced breast cancer: a comparison of intermittent and continuous treatment strategies. N Engl J Med. 1987; 317:1490–5.
15. Falkson G, Gelman RS, Pandya KJ, Osborne CK, Tormey D, Cummings FJ, et al. Eastern Cooperative Oncology Group randomized trials of observation versus maintenance therapy for patients with metastatic breast cancer in complete remission following induction treatment. J Clin Oncol. 1998; 16:1669–76.
16. French Epirubicin Study Group. Epirubicin-based chemotherapy in metastatic breast cancer patients: role of dose-intensity and duration of treatment. J Clin Oncol. 2000; 18:3115–24.
17. Gennari A, Amadori D, De Lena M, Nanni O, Bruzzi P, Lorusso V, et al. Lack of benefit of maintenance paclitaxel in first-line chemotherapy in metastatic breast cancer. J Clin Oncol. 2006; 24:3912–8.
18. Gligorov J, Doval D, Bines J, Alba E, Cortes P, Pierga JY, et al. Maintenance capecitabine and bevacizumab versus bevacizumab alone after initial first-line bevacizumab and docetaxel for patients with HER2-negative metastatic breast cancer (IMELDA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014; 15:1351–60.
19. Harris AL, Cantwell BM, Carmichael J, Wilson R, Farndon J, Dawes P, et al. Comparison of short-term and continuous chemotherapy (mitozantrone) for advanced breast cancer. Lancet. 1990; 335:186–90.
20. Mayordomo JI, Baena JM, Cirera L, Sanchez-Rovira P, Godes MJ, Galan A, et al. Final results of a randomized trial on the role of maintenance chemotherapy with weekly paclitaxel for patients with metastatic breast cancer. J Clin Oncol. 2009; 15(Suppl):1001.
21. Kloke O, Klaassen U, Oberhoff C, Hartwich G, Szanto J, Wolf E, et al. Maintenance treatment with medroxyprogesterone acetate in patients with advanced breast cancer responding to chemotherapy: results of a randomized trial. Essen Breast Cancer Study Group. Breast Cancer Res Treat. 1999; 55:51–9.
22. Nooij MA, de Haes JC, Beex LV, Wildiers J, Klijn J, Becquart D, et al. Continuing chemotherapy or not after the induction treatment in advanced breast cancer patients. clinical outcomes and oncologists’ preferences. Eur J Cancer. 2003; 39:614–21.
23. Gennari A, Stockler M, Puntoni M, Sormani M, Nanni O, Amadori D, et al. Duration of chemotherapy for metastatic breast cancer: a systematic review and meta-analysis of randomized clinical trials. J Clin Oncol. 2011; 29:2144–9.
24. Yu YF, Wang Y, Fu TP, Chen K, Liu JQ, Yao HR. Trastuzumab combined with doublet or single-agent chemotherapy as first-line therapy for HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2018; 168:337–48.
25. Xie N, Qin T, Ren W, Yao H, Yu Y, Hong H. Efficacy and safety of cyclin-dependent kinases 4 and 6 inhibitors in HR+/HER2− advanced breast cancer. Cancer Manag Res. 2020; 12:4241–50.
26. Jacquet E, Lardy-Cleaud A, Pistilli B, Franck S, Cottu P, Delaloge S, et al. Endocrine therapy or chemotherapy as first-line therapy in hormone receptor-positive HER2-negative metastatic breast cancer patients. Eur J Cancer. 2018; 95:93–101.
27. Sutherland S, Miles D, Makris A. Use of maintenance endocrine therapy after chemotherapy in metastatic breast cancer. Eur J Cancer. 2016; 69:216–22.
28. Kwan TT, Bardia A, Spring LM, Giobbie-Hurder A, Kalinich M, Dubash T, et al. A digital RNA signature of circulating tumor cells predicting early therapeutic response in localized and metastatic breast cancer. Cancer Discov. 2018; 8:1286–99.
29. Yu Y, Zhang W, Li A, Chen Y, Ou Q, He Z, et al. Association of long noncoding RNA biomarkers with clinical immune subtype and prediction of immunotherapy response in patients with cancer. JAMA Netw Open. 2020; 3:e202149.
30. Zhao J, Wang Y, Lao Z, Liang S, Hou J, Yu Y, et al. Prognostic immune-related gene models for breast cancer: a pooled analysis. Onco Targets Ther. 2017; 10:4423–33.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download