Abstract
Purpose
Approximately 30%–40% of pediatric acute myeloid leukemia (AML) patients relapse. In this study, we analyzed the outcome and prognostic factors of relapsed AML patients who had previously received first-line therapy at our institution.
Materials and Methods
The study group consisted of 50 patients who had been diagnosed with AML from April 2009 to December 2018, and then showed first relapse. Thirty-two of the patients (64%) had previously received allogeneic hematopoietic stem cell transplantation (HSCT) in first complete remission (CR).
Results
Forty-five of the patients (90%) received intensive chemotherapy upon diagnosis of relapse, and 76% (34/45) of these patients achieved a second CR. Estimated 5-year overall survival for these 45 patients was 44.9%±7.6%. Time from diagnosis to relapse, extramedullary involvement (EMI) at diagnosis, core binding factor AML, and complex karyotype were significant prognostic factors; in multivariate study, both time from diagnosis to relapse and EMI at diagnosis proved significant. There was no difference in 5-year disease-free survival between patients previously treated with chemotherapy only and those who received HSCT in first CR (52.4%±14.9% vs. 52.6%±11.5%). Of the 19 patients who achieved second CR after previous allogeneic HSCT in first CR and subsequent relapse, 11 were treated with chemotherapy only, and seven survive disease-free.
Despite improvements in survival for pediatric acute myeloid leukemia (AML), relapse remains the most important cause of treatment failure [1]. The long-term overall survival (OS) rate for relapsed patients reported previously was less than 30% [2–4], whereas more recent studies have shown incremental improvement to 30%–40% [5–8]. In contrast to changes in outcome, the key prognostic factors for relapsed AML patients have remained consistent irrespective of study group and period. An aggregate of risk factors reported in these studies predicting better survival include a longer duration from diagnosis to relapse, favorable genetic features of the leukemic blast, prior omission of allogeneic hematopoietic stem cell transplantation (HSCT) in first complete remission (CR), and treatment with allogeneic HSCT in second CR [2–8]. In addition, one study found that the early response of relapsed AML to salvage therapy, as determined by the bone marrow (BM) blast percentage on day 28, was the most significant prognostic factor [9].
With this background, our main objective in this study was to determine outcome and important prognostic factors for relapsed pediatric AML patients diagnosed during a period of 10 years at our institution.
The study received approval from our institutional review board. Patients diagnosed with AML at the Department of Pediatrics, The Catholic University of Korea from April 2009 to December 2018, who had received first-line therapy at our institution, and then subsequently relapsed were included. Primary refractory patients, and those diagnosed with a non-AML secondary malignancy after initial diagnosis and treatment for AML were excluded. The final study group consisted of 50 relapsed patients (Table 1). Seventeen of the patients had previously been reported as part of different studies [10,11].
The median age at diagnosis was 10.6 years (range, 0.5 to 18.8 years). The most common genetic abnormality was RUNX1-RUNX1T1 fusion, detected in 13 patients (26%). Seventeen patients (34%) had extramedullary involvement (EMI) at diagnosis, including leukemic involvement confirmed through cerebrospinal fluid study (n=3) and EMI diagnosed through imaging (n=14). Six of 13 patients with RUNX1-RUNX1T1 fusion had EMI diagnosed through imaging, as well as two of three patients with −5/del(5q) abnormality. The remaining patients with imaging-based EMI had the following genetic abnormalities: complex karyotype (n=2), normal karyotype (n=2), RBM15-MKL1 (n=1), and other non-complex (n=1).
The first-line chemotherapy regimens that the patients received were reported previously [11]. Patients diagnosed from 2008 to 2011 received Regimen 2008, while those diagnosed from 2012 onwards received AML 2012 chemotherapy as part of a multi-center clinical trial. Both treatment regimens classified patients into three risk groups (broadly low-, intermediate- and high-risk groups) on the basis of genetic abnormalities of the leukemic blast and response to initial chemotherapy. All patients in Regimen 2008 and intermediate-risk patients in AML 2012 underwent allogeneic HSCT in first CR if they had a human leukocyte antigen (HLA) matched donor. All high-risk patients in AML 2012 received allogeneic HSCT in first CR, regardless of presence of an HLA-matched donor. In the relapse study group, initial risk group classification was as follows: low eight (16%), intermediate 19 (38%), high 23 (46%). Thirty-two patients (64%) had received allogeneic HSCT in first CR.
Of the 14 core binding factor (CBF) AML patients, eight initially received chemotherapy only: for Regimen 2008, three low-risk patients who did not have an HLA-matched donor; for AML 2012, four intermediate-risk patients (KIT mutation (+) [n=3], delayed CR [n=1]) who did not have an HLA-matched donor, and one low-risk patient. Six patients received allogeneic HSCT in first CR: for Regimen 2008, two low-risk patients with an HLA-matched donor; for AML 2012, three intermediate-risk patients (KIT mutation (+)) with an HLA-matched donor, and one high-risk patient (concurrent FMS-like tyrosine kinase 3 [FLT3]–internal tandem duplication [ITD] mutation (+)).
Key objectives of the study were to initially determine the estimated probability of OS in the main study group of 50 patients. A second objective was to determine OS and risk factors for OS in the subgroup of patients who initially received intensive chemotherapy after diagnosis of relapse. We analyzed the influence of the following risk factors determined at initial diagnosis of AML in these patients on OS: patient sex, age at diagnosis, initial white blood cell (WBC) count, EMI at diagnosis, CBF AML, presence of FLT3-ITD, complex karyotype, chemotherapy regimen, achievement of first CR after 1 course of remission induction chemotherapy, allogeneic HSCT in first CR. We further analyzed the impact of the following variables on OS: period of relapse and time from diagnosis to relapse. We also attempted to determine whether the following relapse-specific variables affected OS: age and WBC count at relapse, as well as, CBF AML, FLT3-ITD mutation, complex karyotype at relapse. We also calculated the disease-free survival (DFS) for patients who achieved a second CR, and the OS and DFS of the two subgroups of patients who either relapsed after receiving chemotherapy only, or relapsed after treatment with allogeneic HSCT in first CR. Finally, we calculated the event-free survival (EFS) for patients who underwent post-relapse HSCT, and compared outcome according to type of conditioning regimen.
Comparison of key prognostic factors at relapse (WBC count at relapse, and genetic abnormalities at relapse [CBF AML, FLT3-ITD, complex karyotype status]) between those who received chemotherapy only in first CR, and those who received allogeneic HSCT in first CR was done with the Mann-Whitney and chi-square tests. OS was determined from the date of relapse to death or last follow-up, while DFS was determined from the date of second CR to subsequent relapse, death, or last follow-up. The EFS of the patients who received post-relapse HSCT was calculated from the time of HSCT to relapse, death, or last follow-up. OS, DFS, and EFS were calculated with the Kaplan-Meier method. Univariate and multivariate study of risk factors for OS were done with the log-rank test and Cox proportional hazard regression, respectively. Patient follow-up was done up till December 31, 2020. p-values < 0.05 were considered significant.
For the overall study group, the median time from diagnosis to relapse was 12.2 months (range, 2.8 to 62.2 months). Sites of relapse were as follows: BM 41, extramedullary (EM) 2, BM and EM combined 7. Although 34 of 48 evaluable patients (71%) showed cytogenetic changes of the leukemic blast from diagnosis to relapse, changes in the recurrent genetic abnormalities with prognostic relevance were only found in six patients: normal karyotype to BCR-ABL1 (n=1), FLT3-ITD (+) to FLT3-ITD (−) (n=1), complex karyotype to monosomy 7 (n=1), complex karyotype to non-complex karyotype (n=2), non-complex karyotype to complex karyotype (n=1). One patient with concurrent RUNX1-RUNX1T1 fusion and FLT3-ITD mutation showed loss of FLT3-ITD mutation at relapse but retained the key RUNX1-RUNX1T1 fusion.
Five patients did not begin treatment with intensive chemotherapy upon diagnosis of relapse: two patients who did not receive curative therapy and failed to achieve second CR, two patients who reached second CR after decrease and cessation of immunosuppression only, and one patient who received local radiotherapy only for treatment of isolated EM relapse.
Of the 45 patients who received intensive chemotherapy, 41 patients were treated with a combination of fludarabine, cytarabine, and granulocyte colony stimulating factor with or without idarubicin (FLAG±IDA) as the first reinduction regimen (Table 2). FLAG±IDA resulted in second CR in 28 patients (68%), while none of the other four reinduction strategies resulted in second CR. The rate of second CR after the first course of chemotherapy was 62% (28/45), while the final, overall rate of second CR was 76% (34/45). Six patients achieved second CR after more than one salvage attempt, including four patients after the second reinduction, and one patient who showed CR of EM relapse after three courses of chemotherapy. One patient who failed to achieve second CR after two courses of reinduction chemotherapy went on to receive a second allogeneic HSCT without remission, and achieved second CR after transplant.
For the overall study group of 50 patients, the estimated probability of 5-year OS was 40.1%±7.1% (20/50) (Fig. 1). Among the subgroup of 45 patients who received initial intensive chemotherapy upon diagnosis of relapse, 11 pati-ents died during reinduction chemotherapy or from refractory disease, and failed to achieve second CR. Among the 34 patients who achieved second CR, 13 patients experienced a second relapse, and two patients died from treatment-related causes in second CR, resulting in a 5-year DFS of 52.8%±9.2% (19/34) (Fig. 2A). The 5-year OS for the 45 patients treated with initial intensive chemotherapy was 44.9%±7.6% (20/45) (Fig. 2B), with a median duration of follow-up of 36.6 months (range, 6.0 to 111.2 months) for those who achieved a second CR.
Regarding risk factors for OS in these 45 patients determined at the time of diagnosis, time from diagnosis to relapse, EMI at diagnosis, presence of CBF AML and presence of complex karyotype proved significant (Table 3, Fig. 3A–D). When undertaking multivariate study with the two most significant variables, time from diagnosis to relapse and EMI at diagnosis, both factors proved significant (time from diagnosis to relapse: hazard ratio [HR], 2.66; 95% confidence interval [CI], 1.13 to 6.28; p=0.025; EMI at diagnosis: HR, 2.33; 95% CI, 1.02 to 5.31; p=0.044).
Regarding risk factors determined at the time of relapse, persistent CBF AML and FLT3-ITD mutation at relapse were significant factors influencing OS (S1 Table). In multivariate study, FLT3-ITD mutation at relapse was significant (HR, 3.35; 95% CI, 1.19 to 9.43; p=0.022).
Of the 45 patients who received intensive reinduction chemotherapy upon relapse, 18 had been previously treated with chemotherapy only without HSCT (Fig. 4). Fifteen of these 18 patients achieved second CR (83%), all of whom proceeded to allogeneic HSCT. For these 15 patients, the median number of post-relapse chemotherapy courses prior to HSCT was 2 (range, 2 to 3), and the median time from relapse to HSCT was 3.5 months (range, 2.4 to 4.6 months). Four patients relapsed post-HSCT and two patients died of treatment-related causes in CR, resulting in a 5-year DFS of 52.4%±14.9% (9/15). For the overall subgroup of 18 patients treated with chemotherapy only prior to relapse, the 5-year OS was 50.8%±12.9% (9/18).
Twenty-seven of 45 patients who received intensive chemotherapy upon relapse had received allogeneic HSCT in first CR. The FLT3-ITD mutation was found at relapse solely in these patients who had received HSCT in first CR, while there was no difference in other key prognostic factors found at relapse when comparing patients who had received HSCT in first CR with those who had received chemotherapy only in first CR (S2 Table).
Nineteen of 27 patients achieved second CR (70%), and there was no difference in 5-year DFS when comparing the HSCT in first CR and chemotherapy only subgroups (5-year DFS 52.6±11.5% vs. 52.4±14.9%, p=0.572). Four of these 19 patients proceeded to second allogeneic HSCT in second CR at a median time from relapse to HSCT of 4.2 months (range, 3.5 to 5.8); of these patients, one patient relapsed and the remaining three patients survive without event.
Of the remaining 15 patients, 14 were treated with chemotherapy only, either completing the planned treatment (n=11), or until early second relapse (n=3), while one patient received a second allogeneic HSCT without CR.
For the 11 patients who completed treatment with chemotherapy only, the median number of chemotherapy courses was 4 (range, 2 to 4). Seven survived without further event, and the genetic abnormalities of these patients at initial diagnosis were as follows: RUNX1-RUNX1T1 (n=3), FLT3-ITD (n=2), and normal karyotype (n=2) (Table 4). For these seven patients, changes in key genetic abnormalities at relapse were observed in two patients (FLT3-ITD (+) with normal karyotype to FLT3-ITD (−) with non-complex karyotype [n=1] and normal karyotype to non-complex karyotype [n=1]), resulting in the following at relapse: RUNX1-RUNX1T1 (n=3), non-complex karyotype (n=2), FLT3-ITD (+) (n=1), and normal karyotype (n=1). The one patient who showed persistent FLT3-ITD mutation at relapse survives disease-free after 4 cycles of FLAG chemotherapy without FLT3 inhibitor therapy.
For the overall subgroup of patients who relapsed post-allogeneic HSCT in first CR, the 5-year OS was 40.7%±9.5% (11/27), with no difference in OS when compared with patients treated with chemotherapy only prior to relapse (p=0.617).
Overall, 20 patients (40%) received allogeneic HSCT after relapse at a median of 3.7 months from relapse (range, 2.4 to 5.8 months), 15 as the first transplant, and five as the second transplant after receiving HSCT in first CR. A haploidentical family donor (HFD) was utilized in 15 of 20 HSCTs (Table 5), with rabbit anti-thymocyte globulin (ATG, thymoglobuline, Sanofi, Paris, France)-based T cell depletion given in 13 patients, and post-transplantation cyclophosphamide in the remaining two patients. Since 2014, we have utilized a conditioning regimen of total body irradiation (TBI) 800 cGy, busulfan (Bu, 130 mg/m2/day for 2 days) and fludarabine (Flu, 40 mg/m2/day for 4 days) for relapsed AML patients, whereas the previous conditioning regimen consisted of Bu (130 mg/m2/day for 4 days) and Flu (40 mg/m2/day for 4 days). Patients who received a TBI-Bu-Flu regimen had better outcome than those who received the previous Bu-Flu regimen (5-year EFS, 72.5%±14.1% for TBI-Bu-Flu vs. 28.6%±17.1% for Bu-Flu; p=0.041), although the different doses of ATG administered for each conditioning regimen may also have influenced transplant outcome (Table 5). Evaluating a limited number of patients, those who received a matched sibling donor or matched unrelated donor HSCT had better 5-year EFS than those who received an HFD HSCT (80.0%±17.9% vs. 47.6%±14.3%, p=0.369).
Of the 34 patients who achieved second CR, 28 patients reached CR after the first course of reinduction chemotherapy, while 32 patients overall achieved CR within two courses of reinduction chemotherapy. Hence, the vast majority of patients who achieved second CR did so within the initial attempts of salvage chemotherapy, as reported previously for relapsed or refractory AML [13].
Several reinduction regimens may be given for relapsed AML, with none being standard therapy. The majority of patients in our study received FLAG±IDA, the efficacy of which has been shown for relapsed patients [14]. This reinduction strategy resulted in second CR in 68% of patients treated with FLAG±IDA. Utilization of targeted or novel therapies such as gemtuzumab ozogamicin or venetoclax may further increase the rate of second CR [15,16].
In terms of outcome, the 5-year OS rates of 40.1%±7.1% and 44.9%±7.6% for the overall study group and for those who received reinduction chemotherapy, respectively were similar to those reported in more recent studies [5–7]. Time from diagnosis to relapse was the most significant prognostic factor for OS, reflecting consensus on the key role of this variable in post-relapse survival [2–7].
Of note, we found that patients with EMI at diagnosis had significantly worse outcome than those who lacked EMI. Studies on the role of EMI in pediatric AML outcome are conflicting. Recent studies based on a large number of patients showed that patients with EMI had a higher risk of induction death, and that EMI was not a prognostic factor for patients who undergo HSCT [17,18]. The incidence of EMI at diagnosis in our study group was 34%, higher than the 23% found in both recent studies [17,18]. Our strategy of active imaging-based surveillance for EMI at diagnosis as reported previously may have contributed to a greater incidence of EMI in our study group [11].
In terms of genetic abnormalities, we were able to confirm improved outcome in relapsed CBF AML patients, as shown in previous studies [5,6]. Furthermore, patients with a complex karyotype had lower survival compared with those lacking this genetic abnormality. Although past studies have shown that high-risk genetic features at diagnosis, such as the FLT3-ITD mutation, may result in poor outcome after relapse [6,19], none has focused on the prognostic role of complex karyotype in relapsed AML. The potential adverse effect of a complex karyotype on outcome of newly diagnosed pediatric AML lacks the consensus observed for other poor prognosis genetic abnormalities. However, a study of 454 pediatric AML patients showed that patients with a complex karyotype had significantly worse EFS than other patients [20]. Further study with a larger number of patients is necessary to confirm whether novel prognostic factors in the relapsed pediatric AML setting, such as EMI or a complex karyotype at diagnosis, define a high risk group of patients with inferior outcome after relapse. Regarding the influence of relapse-specific variables on patient outcome, persistent FLT3-ITD mutation at relapse predicted worse OS in multivariate study, consistent with the established poor prognosis of this genetic abnormality.
The data in this study derive from patients initially treated for AML using two consecutive, institutional protocols, Regimen 2008 and AML 2012 [11]. In both of these treatment regimens, many high-risk patients underwent allogeneic HSCT in first CR, while intermediate-risk patients also received allogeneic HSCT if they had HLA-matched donors. Hence, in our relapsed patient study group, the majority of patients (64%) had received allogeneic HSCT in first CR, in contrast to recent studies on relapsed, pediatric AML in which the proportion of patients who had received HSCT in first CR was a clear minority [5,7].
In the subgroup of patients who had been treated with chemotherapy only prior to relapse, all patients who achieved second CR proceeded to HSCT after a median of two chemotherapy courses. A previous study also showed that relapsed AML patients who received 2 cycles of pre-transplant chemotherapy had better OS than those who received 1 or 3 or more cycles of chemotherapy, possibly by lowering disease burden while minimizing treatment-related toxicity prior to transplant [21].
For patients who relapse after allogeneic transplant in first CR, curative treatment incorporates a second HSCT. A recent study based on 333 AML children who relapsed after HSCT showed 4-year OS rates of 14% for the entire cohort and 31% for 122 children who received a second HSCT [22]. In our study group, the decision as to whether a patient would proceed to a second transplant was individualized, rather than based on a pre-planned strategy. Factors contributing to the decision were our attempts to minimize the number of patients who proceeded to second transplant during the treatment period of our study, due mostly to concern for late effects. We also aimed to forgo a second transplant for the good prognosis CBF AML patients who had received allogeneic HSCT in first CR.
As a result, in our subgroup of patients who relapsed after allogeneic HSCT in first CR, most of the patients who achieved a second CR completed a salvage strategy based on chemotherapy only (11 of 19 patients), with a median number of four chemotherapy courses, rather than receiving a second HSCT; of these 11 patients, seven survive disease-free. Some of the patients who were cured with a chemotherapy-only strategy had favorable genetic features, such as RUNX1-RUNX1T1. As these low risk patients would likely not have received allogeneic HSCT in first CR in other cooperative studies, whether this possibility of achieving disease-free status without a second HSCT is specific to our institutional context, rather than being broadly applicable requires further evaluation.
For all relapsed patients, allogeneic HSCT in second CR is a key component of curative therapy. Most of the 20 patients who proceeded to allogeneic HSCT after relapse received an HFD transplant. Also, patients who received a conditioning regimen consisting of TBI-Bu-Flu had better outcome than those who received Bu-Flu, although the differing doses of ATG given in each conditioning regimen confound the comparison. The feasibility of TBI-Bu-Flu in the HFD transplant setting was shown in adult AML patients [23]. Further studies are necessary to confirm the efficacy of this regimen in high-risk or relapsed pediatric AML patients.
Overall, there was no difference in either 5-year OS or DFS between the chemotherapy only and first CR HSCT subgroups who received intensive reinduction chemotherapy, in contrast to previous reports which showed that HSCT prior to relapse had a significant, negative effect on outcome [4,5]. In an earlier study, some of the patients who relapsed early after HSCT received supportive care only rather than intensive chemotherapy, contributing to the discrepancy in survival between post-HSCT and transplant-naïve relapsed patients [4]. Our study supports the role of intensive chemotherapy post-relapse in curing a significant proportion of patients, regardless of prior treatment methods. We also note that with regards to patients who relapsed after allogeneic HSCT, 5-year OS and DFS have improved since our previous report on a historical cohort (OS, 41% vs. 32%; DFS, 53% vs. 33%), with the caveat that some of the patients in this past cohort had received the first HSCT in second CR [10].
We emphasize the main limitations of our single institution study: that is, retrospective in nature, and based on a small number of patients. However, we confirmed the well-established prognostic role of duration from diagnosis to relapse in our relapsed AML study group. Further study is necessary to validate whether factors such as EMI or a complex karyotype at diagnosis can be added to the variables that may affect outcome post-relapse. Our results also indicate that for relapsed pediatric AML patients, intensive therapy may result in long-term survival in 40–50% of patients, and in 50% of patients who achieve second CR, irrespective of prior treatment modalities in first remission.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
The study received approval from the ethical review board of Seoul Saint Mary’s Hospital, The Catholic University of Korea (IRB No. KC20RISI0627). Requirement for patient consent was waived.
ACKNOWLEDGMENTS
We express heartfelt gratitude to the nursing staff associated with the Division of Hematology/Oncology, Department of Pediatrics, The Catholic University of Korea, and, above all, to the patients and their families.
References
1. Rasche M, Zimmermann M, Borschel L, Bourquin JP, Dworzak M, Klingebiel T, et al. Successes and challenges in the treatment of pediatric acute myeloid leukemia: a retrospective analysis of the AML-BFM trials from 1987 to 2012. Leukemia. 2018; 32:2167–77.

2. Webb DK, Wheatley K, Harrison G, Stevens RF, Hann IM. Outcome for children with relapsed acute myeloid leukaemia following initial therapy in the Medical Research Council (MRC) AML 10 trial. MRC Childhood Leukaemia Working Party. Leukemia. 1999; 13:25–31.

3. Rubnitz JE, Razzouk BI, Lensing S, Pounds S, Pui CH, Ribeiro RC. Prognostic factors and outcome of recurrence in childhood acute myeloid leukemia. Cancer. 2007; 109:157–63.

4. Sander A, Zimmermann M, Dworzak M, Fleischhack G, von Neuhoff C, Reinhardt D, et al. Consequent and intensified relapse therapy improved survival in pediatric AML: results of relapse treatment in 379 patients of three consecutive AML-BFM trials. Leukemia. 2010; 24:1422–8.

5. Karlsson L, Forestier E, Hasle H, Jahnukainen K, Jonsson OG, Lausen B, et al. Outcome after intensive reinduction therapy and allogeneic stem cell transplant in paediatric relapsed acute myeloid leukaemia. Br J Haematol. 2017; 178:592–602.

6. Moritake H, Tanaka S, Miyamura T, Nakayama H, Shiba N, Shimada A, et al. The outcomes of relapsed acute myeloid leukemia in children: results from the Japanese Pediatric Leukemia/Lymphoma Study Group AML-05R study. Pediatr Blood Cancer. 2021; 68:e28736.

7. Rasche M, Zimmermann M, Steidel E, Alonzo T, Aplenc R, Bourquin JP, et al. Survival following relapse in children with acute myeloid leukemia: a report from AML-BFM and COG. Cancers (Basel). 2021; 13:2336.

8. Kaspers GJ, Zimmermann M, Reinhardt D, Gibson BE, Tamminga RY, Aleinikova O, et al. Improved outcome in pediatric relapsed acute myeloid leukemia: results of a randomized trial on liposomal daunorubicin by the International BFM Study Group. J Clin Oncol. 2013; 31:599–607.

9. Creutzig U, Zimmermann M, Dworzak MN, Gibson B, Tamminga R, Abrahamsson J, et al. The prognostic significance of early treatment response in pediatric relapsed acute myeloid leukemia: results of the international study Relapsed AML 2001/01. Haematologica. 2014; 99:1472–8.

10. Lee JW, Jang PS, Chung NG, Cho B, Kim HK. Treatment of children with acute myeloid leukaemia who relapsed after allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2013; 160:80–6.

11. Lee JW, Kim S, Jang PS, Chung NG, Cho B, Im SA, et al. Prognostic role of postinduction minimal residual disease and myeloid sarcoma type extramedullary involvement in pediatric RUNX1-RUNX1T1 (+) acute myeloid leukemia. J Pediatr Hematol Oncol. 2020; 42:e132–9.

12. Ravandi F, Cortes JE, Jones D, Faderl S, Garcia-Manero G, Konopleva MY, et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol. 2010; 28:1856–62.

13. Gorman MF, Ji L, Ko RH, Barnette P, Bostrom B, Hutchinson R, et al. Outcome for children treated for relapsed or refractory acute myelogenous leukemia (rAML): a Therapeutic Advances in Childhood Leukemia (TACL) Consortium study. Pediatr Blood Cancer. 2010; 55:421–9.

14. Fleischhack G, Hasan C, Graf N, Mann G, Bode U. IDA-FLAG (idarubicin, fludarabine, cytarabine, G-CSF), an effective remission-induction therapy for poor-prognosis AML of childhood prior to allogeneic or autologous bone marrow transplantation: experiences of a phase II trial. Br J Haematol. 1998; 102:647–55.

15. Niktoreh N, Lerius B, Zimmermann M, Gruhn B, Escherich G, Bourquin JP, et al. Gemtuzumab ozogamicin in children with relapsed or refractory acute myeloid leukemia: a report by Berlin-Frankfurt-Munster study group. Haematologica. 2019; 104:120–7.

16. Karol SE, Alexander TB, Budhraja A, Pounds SB, Canavera K, Wang L, et al. Venetoclax in combination with cytarabine with or without idarubicin in children with relapsed or refractory acute myeloid leukaemia: a phase 1, dose-escalation study. Lancet Oncol. 2020; 21:551–60.

17. Stove HK, Sandahl JD, Abrahamsson J, Asdahl PH, Forestier E, Ha SY, et al. Extramedullary leukemia in children with acute myeloid leukemia: a population-based cohort study from the Nordic Society of Pediatric Hematology and Oncology (NOPHO). Pediatr Blood Cancer. 2017; 64:e26520.

18. Sakaguchi H, Miyamura T, Tomizawa D, Taga T, Ishida H, Okamoto Y, et al. Effect of extramedullary disease on allogeneic hematopoietic cell transplantation for pediatric acute myeloid leukemia: a nationwide retrospective study. Bone Marrow Transplant. 2021; 56:1859–65.

19. Nakayama H, Tabuchi K, Tawa A, Tsukimoto I, Tsuchida M, Morimoto A, et al. Outcome of children with relapsed acute myeloid leukemia following initial therapy under the AML99 protocol. Int J Hematol. 2014; 100:171–9.

20. von Neuhoff C, Reinhardt D, Sander A, Zimmermann M, Bradtke J, Betts DR, et al. Prognostic impact of specific chromosomal aberrations in a large group of pediatric patients with acute myeloid leukemia treated uniformly according to trial AML-BFM 98. J Clin Oncol. 2010; 28:2682–9.

21. Selim A, Alvaro F, Cole CH, Fraser CJ, Mechinaud F, O’Brien TA, et al. Hematopoietic stem cell transplantation for children with acute myeloid leukemia in second remission: a report from the Australasian Bone Marrow Transplant Recipient Registry and the Australian and New Zealand Children’s Haematology Oncology Group. Pediatr Blood Cancer. 2019; 66:e27812.

22. Uden T, Bertaina A, Abrahamsson J, Ansari M, Balduzzi A, Bourquin JP, et al. Outcome of children relapsing after first allogeneic haematopoietic stem cell transplantation for acute myeloid leukaemia: a retrospective I-BFM analysis of 333 children. Br J Haematol. 2020; 189:745–50.

23. Yahng SA, Kim JH, Jeon YW, Yoon JH, Shin SH, Lee SE, et al. A well-tolerated regimen of 800 cGy TBI-fludarabine-busulfan-ATG for reliable engraftment after unmanipulated haploidentical peripheral blood stem cell transplantation in adult patients with acute myeloid leukemia. Biol Blood Marrow Transplant. 2015; 21:119–29.

Fig. 1
Flow chart of relapsed acute myeloid leukemia study group. CR, complete remission; DFS, disease-free survival; OS, overall survival.
Fig. 2
(A) Estimated disease-free survival (DFS) for the 34 patients who received intensive chemotherapy and achieved second complete remission. (B) Estimated overall survival (OS) for the 45 patients who received intensive chemotherapy.
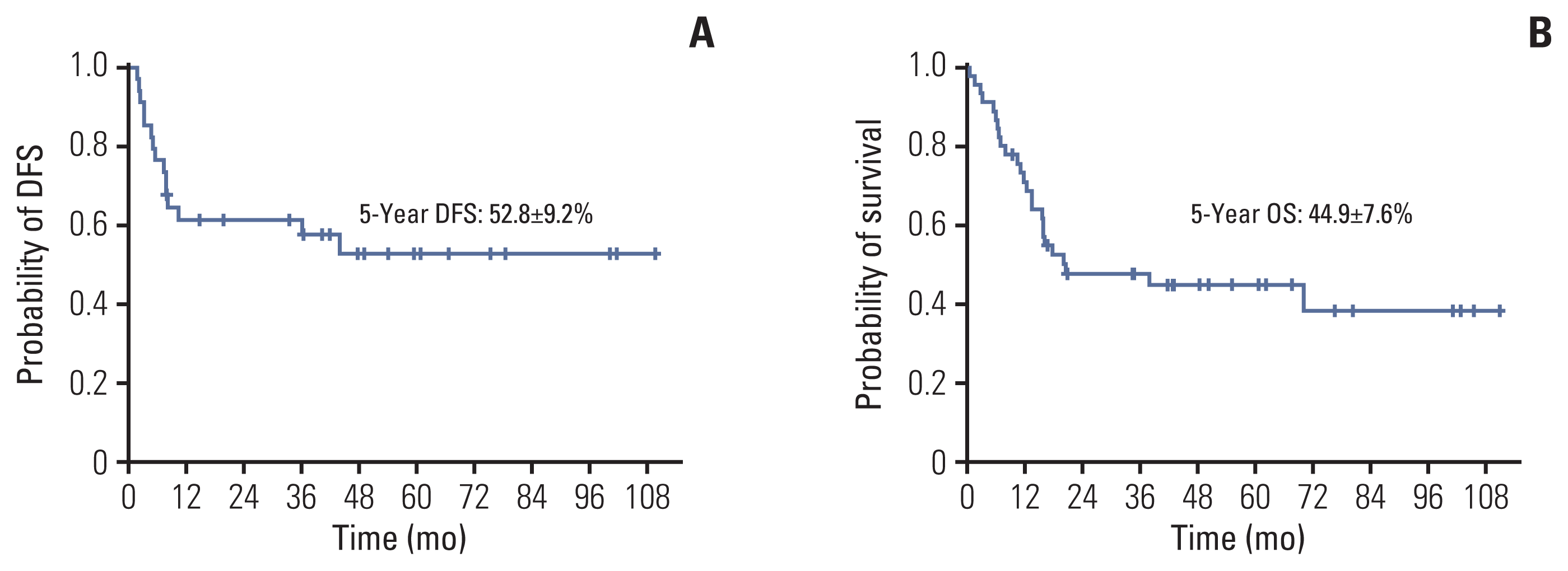
Fig. 3
Estimated overall survival for the 45 patients who received intensive chemotherapy according to time from diagnosis to relapse (< 12 months from diagnosis to relapse vs. ≥ 12 months from diagnosis to relapse) (A), extramedullary involvement (EMI) at diagnosis (B), presence of core binding factor (CBF) acute myeloid leukemia (AML) (C), and presence of complex karyotype (D).
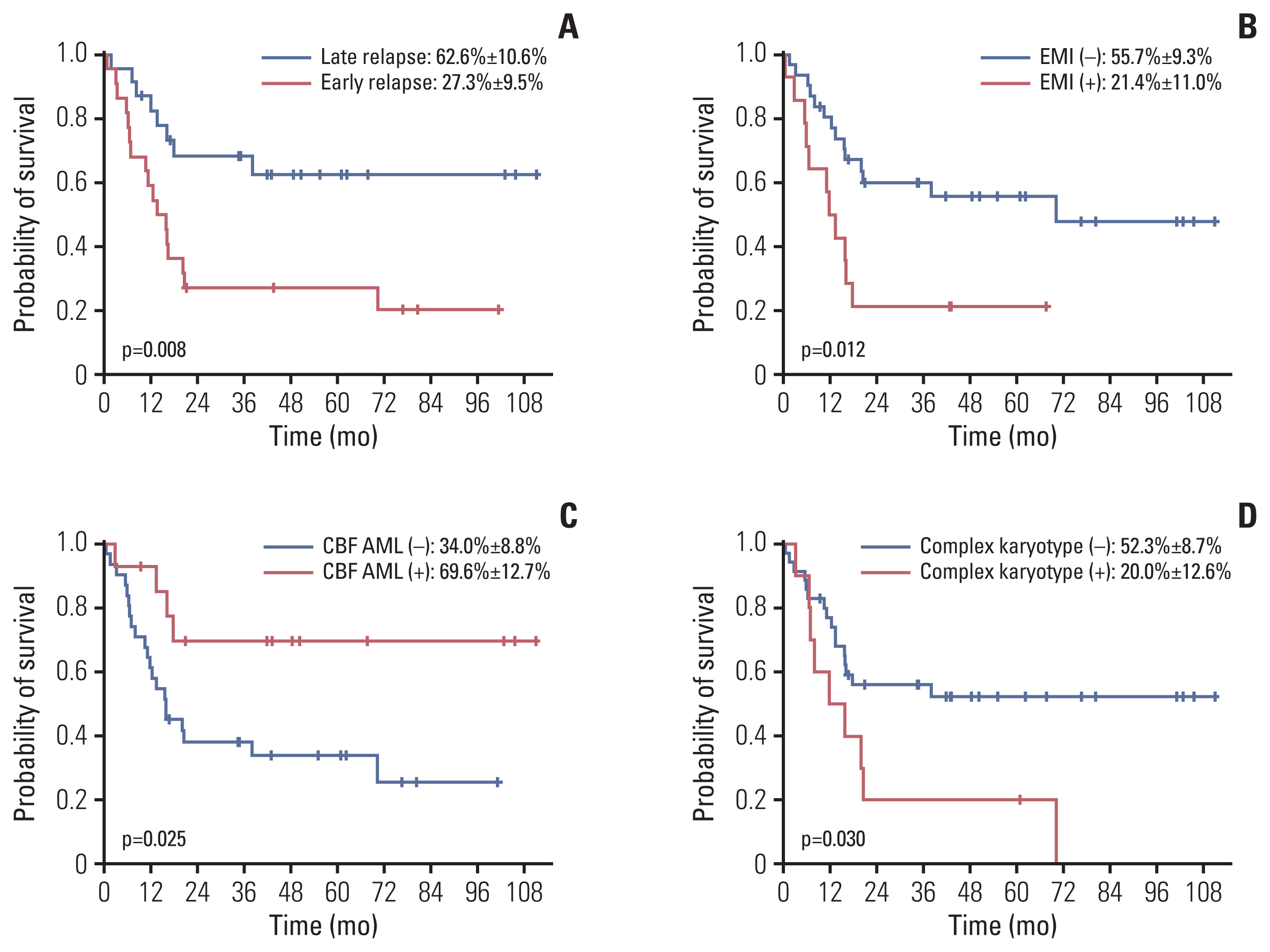
Fig. 4
Disease characteristics, treatment in first complete remission (CR), response to reinduction chemotherapy and outcome for the 45 patients who received intensive chemotherapy. CBF, core binding factor; RI, remission induction. a)Genetic abnormalities at diagnosis of acute myeloid leukemia (AML), b)Achieved complete remission after one course of chemotherapy after initial diagnosis, c)Patient 20 did not achieve second CR after reinduction chemotherapy, and only achieved second CR after the second allogeneic hematopoietic stem cell transplantation (HSCT).
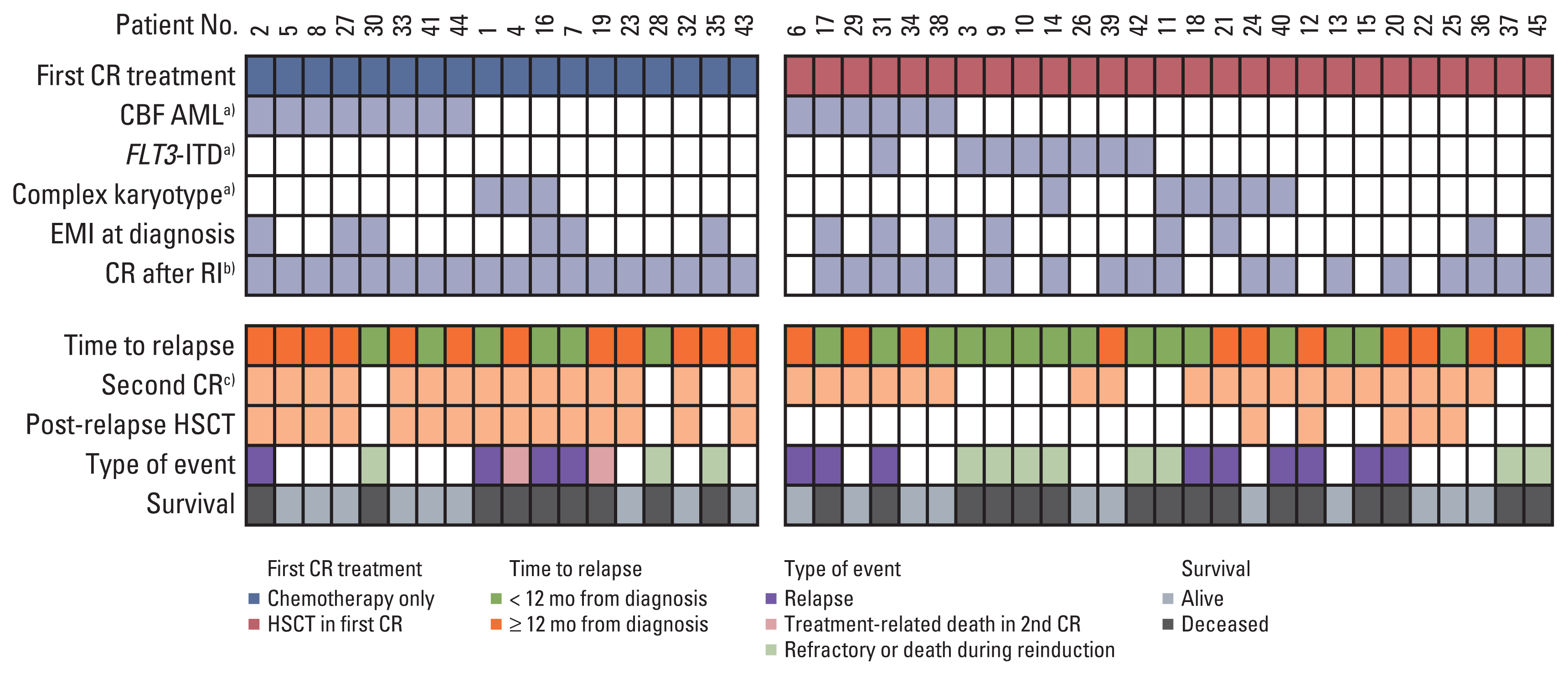
Table 1
Patient characteristics
| Characteristic | No. (%) (n=50) |
|---|---|
| Sex | |
| Male/Female | 31 (62.0)/19 (38.0) |
| Age at diagnosis, median (range, yr) | 10.6 (0.5–18.8) |
| Initial WBC count, median (range, ×109/L) | 17.10 (1.01–287.06) |
| EMI at diagnosisa) | |
| Yes/No | 17 (34.0)/33 (66.0) |
| Genetic abnormalitiesb) | |
| RUNX1-RUNX1T1 | 13 (26.0) |
| FLT3-ITD | 7 (14.0) |
| KMT2A rearrangement | 3 (6.0) |
| −5, del(5q) | 3 (6.0) |
| CBFB-MYH11 | 1 (2.0) |
| DEK-NUP214 | 1 (2.0) |
| FUS-ERG | 1 (2.0) |
| RBM15-MKL1 | 1 (2.0) |
| NPM1 | 1 (2.0) |
| Biallelic CEBPA | 1 (2.0) |
| Other complex karyotypec) | 8 (16.0) |
| Normal | 5 (10.0) |
| Others | 5 (10.0) |
| Initial treatment regimen | |
| Regimen 2008/AML 2012 | 21 (42.0)/29 (58.0) |
| First CR after 1 course of remission induction | |
| Yes/No | 40 (80.0)/10 (20.0) |
| Risk group | |
| Low/Intermediate/High | 8 (16.0)/19 (38.0)/23 (46.0) |
| Allogeneic HSCT in first CR | |
| Yes/No | 32 (64.0)/18 (36.0) |
| Time from diagnosis to relapse, median (range, mo) | 12.2 (2.8–62.2) |
Table 2
Chemotherapy regimens utilized for first reinduction chemotherapy
| Chemotherapy regimen | No. (%) (n=45) |
|---|---|
| Fludarabine 30 mg/m2/day, days 1–5 | 41 (91.1) |
| Cytarabine 2–3 g/m2/day, days 1–5 | |
| G-CSF, days 0–4 | |
| ±Idarubicin 12 mg/m2/day, days 1–3 | |
| IT cytarabine | |
|
|
|
| Cytarabine 2 g/m2 twice daily, days 1–5 | 1 (2.2) |
| Etoposide 100 mg/m2, days 1–5 | |
| IT cytarabine | |
|
|
|
| Cytarabine 3 g/m2 twice daily, days 1–2, 8–9 | 1 (2.2) |
| Asparaginase 6,000 units/m2, days 3, 10 | |
|
|
|
| Cytarabine 3 g/m2 twice daily, days 1–2 | 1 (2.2) |
|
|
|
| Cytarabine 1.5 g/m2/day, days 1–4a) | 1 (2.2) |
| Idarubicin 12 mg/m2/day, days 1–3 | |
| Sorafenib 200 mg/m2 twice daily, days 1–7 | |
a) As detailed in Ravandi et al. [12].
Table 3
Univariate study of risk factors for 5-year OS in patients initially treated with intensive chemotherapy upon relapse diagnosis
| Patients (deceased) | 5-Year OS (±SE) (%) | p-value | |
|---|---|---|---|
| Sex | |||
| Male | 26 (13) | 53.3±9.9 | 0.363 |
| Female | 19 (12) | 31.7±11.7 | |
| Age at diagnosis (yr)a) | |||
| < 11 | 23 (13) | 46.8±10.6 | 0.596 |
| ≥ 11 | 22 (12) | 42.6±11.1 | |
| WBC at diagnosis (×109/L)a) | |||
| < 17 | 22 (11) | 54.5±10.6 | 0.539 |
| ≥ 17 | 23 (14) | 35.5±10.5 | |
| EMI at diagnosis | |||
| No | 31 (14) | 55.7±9.3 | 0.012 |
| Yes | 14 (11) | 21.4±11.0 | |
| CBF AML | |||
| No | 31 (21) | 34.0±8.8 | 0.025 |
| Yes | 14 (4) | 69.6±12.7 | |
| FLT3-ITD | |||
| No | 37 (19) | 49.4±8.5 | 0.057 |
| Yes | 8 (6) | 25.0±15.3 | |
| Complex karyotype | |||
| No | 35 (16) | 52.3±8.7 | 0.030 |
| Yes | 10 (9) | 20.0±12.6 | |
| Chemotherapy regimen | |||
| Regimen 2008 | 17 (13) | 29.4±11.1 | 0.051 |
| AML 2012 | 28 (12) | 54.3±9.9 | |
| First CR after 1 course of remission induction | |||
| No | 9 (6) | 33.3±15.7 | 0.670 |
| Yes | 36 (19) | 47.9±8.6 | |
| HSCT in first CR | |||
| No | 18 (9) | 50.8±12.9 | 0.617 |
| Yes | 27 (16) | 40.7±9.5 | |
| Period of relapse | |||
| 2010–2014 | 23 (17) | 30.4±9.6 | 0.072 |
| 2015–2020 | 22 (8) | 62.2±10.6 | |
| Time from diagnosis to relapsea) | |||
| < 12 mo | 22 (17) | 27.3±9.5 | 0.008 |
| ≥ 12 mo | 23 (8) | 62.6±10.6 |
Table 4
Key characteristics of the patients who relapsed after allogeneic HSCT in first CR and survive disease-free after a chemotherapy only strategy
| Patient No. | EMI at diagnosis | Time from diagnosis to relapse (mo) | Genetics at diagnosis | Genetics at relapse | GVHD after relapse | Treatment after relapse | Survival (mo)a) |
|---|---|---|---|---|---|---|---|
| 13 | No | 10.7 | Normal karyotype | Normal karyotype | No | FLAG-Ida → FLAG (×2) | 102 |
| 26 | No | 9.4 | FLT3-ITD (+) | FLT3-ITD (−) | No | FLAG-Ida (×2) → FLAG (×2) | 77 |
| 29 | No | 23.4 | RUNX1-RUNX1T1 | RUNX1-RUNX1T1 | No | FLAG (×4) | 49 |
| 34 | No | 23.1 | RUNX1-RUNX1T1 | RUNX1-RUNX1T1 | No | FLAG (×4) | 42 |
| 36 | Yes | 14.3 | Normal karyotype | Non-complex karyotype | Yes | FLAG (×2) | 43 |
| 38 | Yes | 8.6 | RUNX1-RUNX1T1 | RUNX1-RUNX1T1 | No | FLAG-Ida → FLAG (×3) | 43 |
| 39 | No | 15.5 | FLT3-ITD (+) | FLT3-ITD (+) | Yes | FLAG (×4) | 35 |
Table 5
Characteristics of post-relapse allogeneic HSCT
| No. (%) (n=20) | |
|---|---|
| Disease status | |
| Second CR | 19 (95.0) |
| Relapsed | 1 (5.0) |
| Donor type | |
| MSD | 2 (10.0) |
| MUD | 3 (15.0) |
| HFD | 15 (75.0) |
| Cell source | |
| BM | 2 (10.0) |
| PBSC | 18 (90.0) |
| Conditioning intensity | |
| Myeloablative | 20 (100) |
| Conditioning type | |
| Bu-Flu-ATGa) | 7 (35.0) |
| TBI-Bu-Flu±(ATG or PTCy)b) | 13 (65.0) |
ATG, anti-thymocyte globulin; BM, bone marrow; Bu, busufan; CR, complete remission; Flu, fludarabine; HFD, haploidentical family donor; HSCT, hematopoietic stem cell transplantation; MSD, matched sibling donor; MUD, matched unrelated donor; PBSC, peripheral blood stem cells; PTCy, post-transplantation cyclophosphamide; TBI, total body irradiation.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download