Increased awareness of this aggressive tumor entity is crucial due to difficulties in initial diagnosis of GEA compared with HPV-associated UEA. Therefore, to improve the cytologic recognition of GEA, the cytological findings of both HPV-independent GEA and HPV-associated UEA were analyzed in the present study.
MATERIALS AND METHODS
Case selection and HPV testing
After obtaining Institutional Review Board approval (CNUH-IRB 2021-12-001) of the Chungnam National University Hospital (CNUH) (Daejeon, Republic of Korea), the pathology database was searched for GEA and UEA diagnoses in cytological and/or surgical pathology specimens at CNUH from July 2016 to June 2021. Clinicopathologic findings and follow-up results were obtained from the electronic medical records (
Table 1).
Table 1
Clinicopathologic findings of GEA and UEA
|
Clinicopathologic findings |
GEA (n = 10) |
UEA (n = 12) |
|
Age (yr) |
|
Mean ± SD |
61.7 ± 11.9 |
45.3 ± 10.9 |
|
Range |
48–80 |
31–68 |
|
Type of surgical treatment |
|
Radical hysterectomy |
7 (70.0) |
8 (66.7) |
|
Total hysterectomy |
0 |
3 (25.0) |
|
Trachelectomy |
1 (10.0) |
0 |
|
Conization |
2 (20.0) |
1 (8.3) |
|
FIGO stage |
|
I |
1 (10.0) |
9 (75.0) |
|
II |
4 (40.0) |
1 (8.3) |
|
III |
3 (30.0) |
2 (16.7) |
|
IV |
2 (20.0) |
0 |
|
Human papillomavirus status |
|
High-risk HPV |
0 |
12 (100) |
|
Not detected |
10 (100) |
0 |
|
p16 expression |
|
Block positive |
1 (10.0) |
12 (100) |
|
Patchy positive/negative |
9 (90.0) |
0 |
|
p53 expression |
|
Diffuse strong positive |
5 (50.0) |
0 |
|
Complete loss (null) |
1 (10.0) |
0 |
|
Patchy positive |
4 (40.0) |
12 (100) |
|
Follow-up results |
|
No evidence of disease |
2 (20.0) |
9 (75.0) |
|
Alive with disease |
3 (30.0) |
0 (0.0) |
|
Died of disease |
4 (40.0) |
0 (0.0) |
|
Died of other disease |
0 |
1a (8.3) |
|
Not available |
1 (10.0) |
2 (16.7) |

A total of 10 GEA cases were included in the study (
Table 2). Diagnosis of GEA was based on the morphologic criteria of GEA [
1,
2] (
Fig. 1A–F) and negative HPV test results. For comparison, 12 control cases of UEA from the same pathology file were also reviewed. These cases were histopathologically diagnosed based on the morphologic criteria of UEA (
Fig. 2A), a positive high-risk HPV test, and block-type positivity for p16 (
Fig. 2B). Immunostained slides were retrieved from the pathology archives and the authors reviewed the slides or pathology reports.
 | Fig. 1Human papillomavirus-independent gastric-type endocervical adenocarcinoma. Irregular angulated glands invading the cervical stroma (A). Tumor cells show negative immunohistochemical reactions for p16 (B), aberrant nuclear overexpression for p53 (C), and positivity for MUC6 (D). Atypical glands with voluminous clear cytoplasm and distinct cell borders (E). The cytoplasm contains neutral mucins that stain pale pinkish-red on Alcian blue/periodic acid–Schiff special staining in contrast to the dark purple of acid mucins of the normal endocervix (inset) (F). 
|
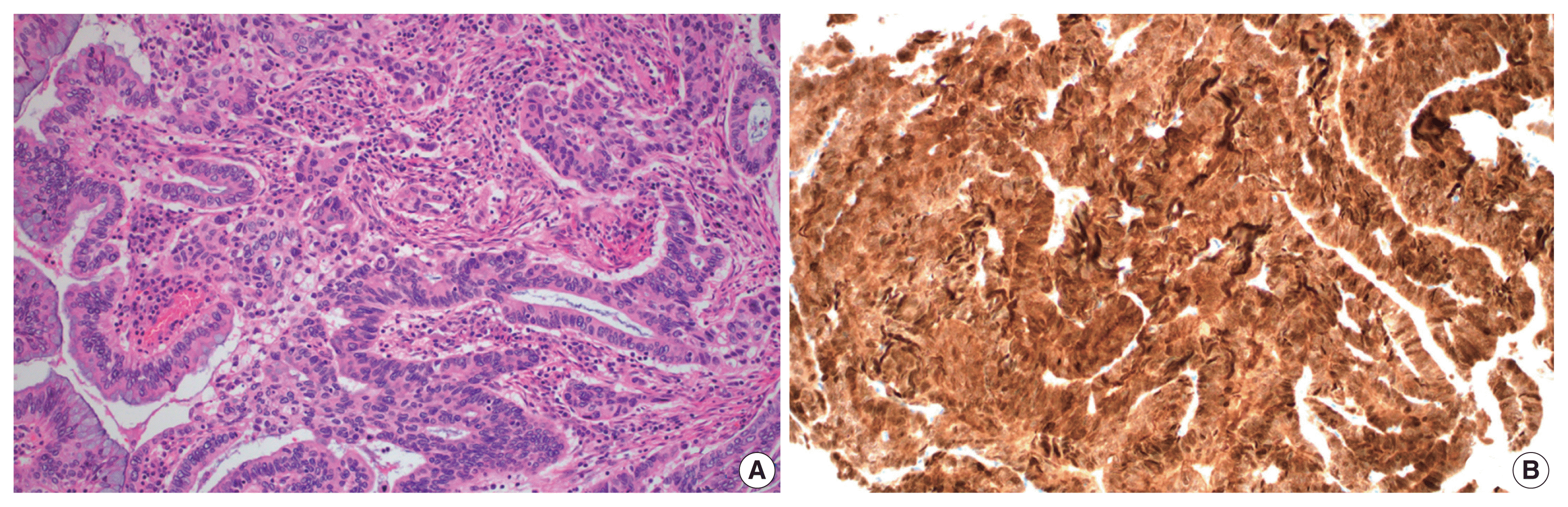 | Fig. 2Human papillomavirus–associated usual-type endocervical adenocarcinoma. Irregular confluent glands composed of mucin-depleted cells with pseudostratified, hyperchromatic nuclei (A). Tumor cells show a block-type immunohistochemical reaction for p16 (B). 
|
Table 2
Clinical and immunohistochemical findings of 10 cases of gastric-type endocervical adenocarcinoma
|
Case No. |
Age (yr) |
Preparation type |
Cytologic diagnosis |
Type of surgery |
FIGO stage |
HPV |
p16 |
p53 |
MUC-6 |
Adjuvant treatment |
Follow-up (mo) |
Outcome |
|
|
CS |
LBP |
|
1 |
48 |
+ |
+ |
AIS |
Trachelectomy |
IVA |
− |
− |
+ |
+ |
CC |
20 |
DOD |
|
2 |
80 |
+ |
+ |
Adenocarcinoma |
RH |
IIB |
− |
− |
Wild |
−a
|
RT |
38 |
DOD |
|
3 |
52 |
+ |
+ |
AGC |
RH |
IIIC1 |
− |
− |
Wild |
+ |
CCRT |
48 |
AWD |
|
4 |
58 |
+ |
+ |
Adenocarcinoma |
RH |
IVA |
−b
|
− |
+ |
+ |
CC |
23 |
DOD |
|
5 |
54 |
+ |
+ |
Adenocarcinoma |
RH |
IIA1 |
− |
+ |
+ |
+ |
NA |
FU loss |
NA |
|
6 |
54 |
NA |
NA |
NA |
Conization |
IIIC1 |
− |
− |
Wild |
+ |
CCRT |
17 |
DOD |
|
7 |
78 |
NA |
+ |
Adenocarcinoma |
Conization |
IIA2 |
− |
− |
+ |
−a
|
RT |
21 |
NED |
|
8 |
63 |
NA |
+ |
AGC |
RH |
IB1 |
− |
− |
Null |
+ |
CCRT |
21 |
NED |
|
9 |
76 |
NA |
+ |
AGC |
RH |
IIB |
− |
− |
+ |
+ |
RT |
10 |
AWD |
|
10 |
54 |
NA |
NA |
NA |
RH |
IIIC1 |
− |
− |
Wild |
+ |
CCRT |
10 |
AWD |

A total of 19 patients (9 GEA and 10 UEA) had HPV based on PANA RealTyper HPV kit (PANA RealTyper, PANAGENE, Daejeon, Korea) results. The remaining one GEA case and two UEA cases had in situ hybridization test results for high-risk HPV using paraffin blocks.
Cytologic examination
Among 10 GEA patients, two patients (cases Nos. 6 and 10) had no Papanicolaou (Pap) test results. Pathologists with expertise in cytopathology and gynecologic pathology (M.K.Y., G.E.B., and K.S.S.) reviewed preoperative cervical smears from the eight GEA patients and 12 UEA patients. All cytologic slides were prepared using the conventional method and/or the liquid-based preparation (LBP; ThinPrep Pap test, Hologic, Bedford, MA, USA), and Pap staining was performed. Both conventional smear and LBP were performed in five GEA cases and nine UEA cases. Only LBP was performed in three GEA cases and one UEA case. Only a conventional smear was performed in two UEA cases. All cytologic results were reported according to The Bethesda System terminology [
7].
Among cytologic features, a mucinous background, architectural (monolayered honeycomb-like sheets, 3-dimensional clusters, picket fence-like feathering), nuclear (vesicular nuclei, hyperchromasia, prominent nucleoli, grooves, intranuclear cytoplasmic pseudoinclusions [INCIs]), and cytoplasmic features (finely vacuolated cytoplasm, cytoplasmic golden-brown mucin, intracytoplasmic neutrophils) were analyzed.
Cytologic findings of the 20 cases (eight GEA and 12 UEA) were scored. A mucinous background and architectural findings were scored as absent, focal (rare or mild degree; 1–2 fields, × 100/slide) or extensive (moderate to high degree; > 3 fields, × 100/slide). Nuclear and cytoplasmic findings were scored as absent, focal (rare or mild degree; < 30% of tumor cells/slide) or extensive (moderate to high degree; > 30% of tumor cells/slide). Cytologic scores were analyzed.
Statistical analysis
All statistical analyses were performed using Statistical Package for Social Sciences Statistics for Windows (SPSS ver. 26.0, IBM Corp., Armonk, NY, USA) software. Groups were compared using Pearson’s χ2 test for categorical variables and p < .05 were considered statistically significant.
Go to :

DISCUSSION
According to a new World Health Organization classification, adenocarcinomas of the uterine cervix are divided into HPV-associated and HPV-independent types with different implications for prognosis [
1]. The HPV-independent category includes gastric, clear cell, mesonephric, and endometrioid types [
1]. GEA, representing the most common HPV-independent adenocarcinoma, is an aggressive tumor type with a poor prognosis regardless of the degree of differentiation [
9]. GEA should not be graded but should be considered high-grade regardless of morphology [
10].
Due to widespread HPV vaccination, the proportion of HPV-independent GEA may increase, rendering recognition of GEA at an early stage even more important [
11]. In the present study, cytological findings of HPV-independent GEA were analyzed and compared with HPV-associated UEA. Monolayered sheets (p = .002) of atypical endocervical cells with vacuolar granular cytoplasm (p = .001) were extensive in GEA; however, three-dimensional clusters (p = .010) were characteristic of UEA.
Kawakami et al. [
12] reviewed the cytologic features of 14 GEA cases compared with 20 UEA control cases based on conventional Pap smear preparations. The characteristic cytologic findings of GEA included monolayered and honeycomb sheets, vacuolar and/or foamy cytoplasm, intracytoplasmic neutrophil entrapment, and vesicular nuclei with prominent nucleoli. Using LBP, tall columnar epithelial cells with pale, foamy or vacuolated cytoplasm were the most common cytologic findings of GEA, followed by well-defined cytoplasmic borders [
11]. Recently, Schwock et al. [
13] reported cytomorphologic features of GEA based on LBP. The most discriminatory findings for GEA versus UEA were microvesicular cytoplasm (100% vs. 17%), honeycomb-like sheets (87% vs. 8%), prominent nucleoli (93% vs. 25%), and anisonucleosis (93% vs. 50%). UEA is cytologically characterized by syncytial aggregates and 3-dimensional clusters of malignant cells with enlarged pleomorphic nuclei, coarse chromatin, macronucleoli, and a finely vacuolated cytoplasm [
7].
In the present study, monolayered and honeycomb sheets of atypical endocervical cells were characteristic findings of GEA cases compared with UEA cases (p = .002). Three-dimensional clusters were significantly more extensive in UEA (11/12) than in GEA patients (3/8, p = .010).
According to Kawakami et al. [
12], a mucinous background was significantly more common in GEA than in UEA cases (p = .024) using the conventional method. In contrast, the so-called “background” extracellular mucin may not be easily detectable in LBP versus direct smears due to preparation-related, technical causes [
13]. Background mucin may clump and cling to tumor cells in LBP instead of the diffuse distribution observed in direct smears [
14]. In general, the diagnostic usefulness of extracellular mucin is doubtful [
13]. In the present study, a background extracellular mucin was identified in 6 GEA and 10 UEA cases. This finding was more extensive in GEA (4/8) than in UEA patients (1/12); however, the difference was not statistically significant (p = .058). The cytologic findings of GEA in LBP compared with conventional smears include cleaner background with reduced background mucin and smaller cellular sheets or clusters. Recently, the diagnostic characteristics to detect GEA using the conventional approach, namely distinct cell borders and prominent nucleoli, were reportedly not useful for excluding UEA in LBP samples. The conventional direct smear provides diagnostic indicators of GEA compared with LBP [
15].
Golden-brown mucin on Pap smears appears to represent a gastric phenotype of endocervical glandular cells that is a unique characteristic shared by MDA and pyloric gland metaplasia. Golden-brown mucin is also present in the context of benign gastric-type glandular proliferations [
16]. On conventional Pap smears, identification of two-toned mucin (yellow gastric-type and pink normal endocervical) has been proposed a diagnostic indicator for lesions with gastric differentiation. However, recognizing yellow mucin on LBP is difficult because the mucin color becomes paler [
17]. Golden-brown mucin may not be a particularly sensitive feature for GEA detection, at least in LBP [
11]. In the present study, two-toned mucin was identified on conventional smears of GEA cases.
Golden-brown intracytoplasmic mucin was occasionally observed in GEA patients (6/14, 42.9%) but observed in only 1 UEA patient (1/20, 5%; p = .034) [
12]. Golden-brown mucin (20%), INCIs (20%), and goblet/Paneth-like cells (20%), although uncommon, represented unique features identified only in GEA [
13]. In the present study, cytoplasmic golden-brown mucin was identified in 5/8 GEA cases and only 2/12 UEA cases; however, the difference was not statistically significant (p = .089).
Intracytoplasmic neutrophil entrapment/phagocytosis was identified in 93% of GEA and 70% of UEA cases (which included endometrial cytology in 12/20 cases). Marked intracytoplasmic neutrophil entrapment was more common in GEA (7/14, 50%) than in UEA cases (2/20, 10%; p = .038) [
12]. In contrast, neutrophil entrapment/phagocytosis was identified in a minority (33%) of both GEA and UEA cases [
13]. In the present study, intracytoplasmic neutrophils among GEA and UEA cases were not statistically significant (p = .461), indicating this feature is of minimal utility for distinguishing between GEA and UEA.
Lobular endocervical glandular hyperplasia (LEGH) is a cervical lesion with pyloric gland metaplasia. Abundant yellow mucin was frequently present in both LEGH and MDA; however, INCIs were found in 22/24 LEGH cases and not found in either MDA or adenocarcinoma cells associated with LEGH [
18]. INCIs were identified in a minority (20%) of GEA cases and not in any UEA case [
13]. In the present study, INCIs were identified in 1 GEA case and 1 UEA case (p = .761) (
Fig. 3D).
Cytologic diagnosis of GEA is problematic and may be difficult to recognize in cytologic specimens. In the present study, preoperative cytologic diagnoses included AGCs, favor neoplastic in three cases, AIS in one case, and adenocarcinoma in four cases. Preoperative cytologic diagnoses of the previously reported GEA cases were reviewed [
11–
13,
15] including the eight cases used in this study (
Table 4). Among a total of 56 cases, the original cytologic interpretations were the following: negative for intraepithelial lesion or malignancy (n = 10, 17.9%); AGC, not otherwise specified/favor neoplastic (n = 16, 28.6%); AIS (n = 3, 5.4%); high-grade squamous intraepithelial lesion (n = 1, 1.8%); adenocarcinoma (n = 25, 44.6%); unsatisfactory for evaluation (n = 1, 1.8%) (
Table 4).
Table 4
Literature review summary of the pretreatment cytologic diagnosis of GEA cases
|
Kawakami et al. [12] |
Lu et al. [11] |
Schwock et al. [13] |
Ryu et al. [15] |
This study |
Total, n (%) |
|
No. of cases |
14 |
11 |
15 |
8 |
8 |
56 (100) |
|
Preparation type |
CS |
LBC |
CS and LBC |
CS and LBC |
CS and/or LBC |
CS and/or LBC |
|
TBS classification |
|
Unsatisfactory |
0 |
1 |
0 |
0 |
0 |
1 (1.8) |
|
NILM |
0 |
5 |
5 |
0 |
0 |
10 (17.9) |
|
AGC |
3 |
4 |
2 |
4 |
3 |
16 (28.6) |
|
AIS |
0 |
0 |
1 |
1 |
1 |
3 (5.4) |
|
HSIL |
0 |
1 |
0 |
0 |
0 |
1 (1.8) |
|
Adenocarcinoma |
11 |
0 |
7 |
3 |
4 |
25 (44.6) |

In cytologic differential diagnosis of GEA, non-neoplastic atypical glandular changes and syncytial aggregates/hyperchromatic crowded groups (HCGs) should also be considered. In patients with an intrauterine device, the endocervical cells are large and have smooth nuclei with smudged chromatin, prominent nucleoli, and occasional nuclear clefts. Large degenerative cytoplasmic vacuoles push the nucleus toward the periphery [
7]. In a reparative process, sheets of atypical endocervical cells have enlarged nuclei with increased nuclear to cytoplasmic ratios, sometimes multiple nucleoli, and mitotic activity. A regular honeycomb arrangement of reactive endocervical cells is also a cytologic feature of non-neoplastic HCGs [
19]. Endocervical or endometrial cells presenting as HCGs may mimic glandular high-grade precancers. Atypical endocervical cells associated with tubal metaplasia can be challenging due to nuclear overlapping and crowding of enlarged, variably sized nuclei; however, identification of cilia and pseudostratified nuclei can be helpful [
7].
In conclusion, GEA can be recognized based on cytologic features of monolayered honeycomb sheets of atypical endocervical cells with abundant vacuolar cytoplasm and some golden-brown intracytoplasmic mucin. In contrast, three-dimensional clusters, feathering, and nuclear hyperchromasia tended to be extensive in UEA. Awareness of the cytomorphologic features of GEA will allow pathologists to recognize and accurately diagnose this rare and aggressive entity.
Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download