This article has been
cited by other articles in ScienceCentral.
Abstract
Heterotopic mesenteric ossification (HMO) is abnormal bone formation in tissues which usually do not undergo ossification. There are approximately 75 cases reported worldwide. We present two cases of HMO. The first case is that of a 39-year-old man who presented with abdominal pain and a computerized tomography scan of the abdomen and pelvis revealed an apple core lesion resulting in small bowel obstruction. The second case is that of a 36-year-old woman who presented 2 months after undergoing robotic gastric sleeve resection complaining of weakness and emesis. An esophagogram revealed kinking at the distal esophagus. Surgical resection was performed in both, yielding the diagnosis of HMO. There are various theories as to the pathophysiology of HMO, but no clearly defined mechanism has been established. Management should be conservative whenever possible to prevent further ossification with subsequent surgical intervention.
Keywords: reports, Heterotopic, Ossification, Mesentery, Stomach, Small bowel
Heterotopic mesenteric ossification (HMO) refers to the abnormal formation of bone in tissues which usually do not undergo ossification. It was first described in 1883 by Riedel, as a sequela after spinal cord injury [
1,
2]. One hundred years later in 1983, Lemeshev et al. [
3] and Hansen et al. [
4] each published a report of heterotopic ossification of the mesentery, and Wilson et al. [
5] coined the term “heterotopic mesenteric ossification” in 1999. Ever since, approximately 75 cases had been reported in literature worldwide [
6–
8]. In most of those cases, an injury, trauma, or surgery to the abdomen preceded the development of HMO [
9]. The latter can occur as early as 11 days or as late as a month after surgery or trauma [
10,
11]. In 1983, Hansen et al. [
4] reported the occurrence of HMO in a 55-year-old man 2 weeks after a coloproctectomy for ulcerative colitis.
Patients usually present with signs and symptoms of small bowel obstruction, such as nausea, vomiting, abdominal pain, and abdominal distension. On imaging, small bowel thickening is commonly seen, and in rare cases, calcifications can be appreciated on computed tomography (CT). There are various theories as to the pathophysiology of this disorder. First, it is postulated that HMO forms as a result of stimulation of pluripotent mesenchymal stem cells due to inflammation from an inciting event, causing differentiation of those stem cells into osteoblasts and osteocytes, aided by osteogenic differentiation factors [
12]. Other factors such as ischemia and infection, which cause inflammation, have been hypothesized as well. Nevertheless, given the rarity and possible underreporting of HMO, no clearly defined mechanism has been established [
13]. Overall, median age at presentation is 48.28 ± 18.27 years, with a male predominance [
8,
14].
Here, we describe two cases of HMO; one is a case of a 39-year-old man who presented with a chief complaint of abdominal pain of 2-day duration. A CT scan of the abdomen and pelvis revealed segmental concentric thickening of a small bowel loop and an apple core lesion resulting in obstruction. Laparoscopic small bowel resection was performed. Histopathologic examination showed an area of mucosal erosion with acute and chronic inflammation in the surrounding peri-intestinal soft and adipose tissue, granulation tissue, fat necrosis, and new bone formation within the mesentery. The second case is that of a 36-year-old woman who underwent gastric sleeve resection and presented two months later complaining of weakness and emesis. An esophagogram demonstrated kinking at the level of distal esophagus at the gastroesophageal junction. Despite multiple attempts with endoscopic interventions and an esophageal stent, the esophageal stricture persisted. Exploratory laparoscopy and an esophagojejunostomy was performed with resection of the esophageal stricture and gastroesophageal junction. Histopathologic examination showed suture granulomas and foreign body giant cell reaction as well as new bone formation within the perigastric tissue. The diagnosis of HMO was made in both cases.
CASE REPORT
Case 1
A 39-year-old man, non-smoker, presented with epigastric abdominal pain of 2-day duration. The pain was sharp in quality, and intermittent in frequency. There were no aggravating or relieving factors. The patient reported one episode of diarrhea but denied fever, chills, nausea, or vomiting. One month prior to his presentation, the patient does admit to having a car accident without any obvious injuries.
The patient had a history of anxiety and panic attacks (on citalopram 20 mg and clonazepam 0.5 mg). There is no significant surgical or family histories and no known allergies. Vital signs on admission were as follows: blood pressure 101/54 mm Hg, pulse 57 beats per minute, respiratory rate 14 per minute, temperature 36°C, and SpO2 97%. Body mass index (BMI) was 28.06 kg/m2. Physical examination revealed a soft abdomen that was tender to deep palpation in the pre-umbilical area with hyperactive bowel sounds.
Laboratory studies showed leukocytosis with a white blood cell count of 13.47 × 10
3/μL (reference range, 4.8 × 10
3/μL to 10.8 × 10
3/μL) and neutrophilia with left shift (80.7% segmented neutrophils (reference range, 42% to 75%). The absolute neutrophil count was 10.87 × 10
3/μL (reference range, 1.8 × 10
3/μL to 7.2 × 10
3/μL), and lymphocytes were decreased at 12.3% (reference range, 16.0% to 45.0%). Red cell indices and electrolytes were within normal limits. Urine toxicology screen was negative. Other relevant laboratory results of patient 1 are summarized in
Table 1.
A CT scan with intravenous contrast of the abdomen and pelvis was performed, showing segmental concentric thickening of the jejunum in the right upper quadrant of the abdomen (with apple core configuration) resulting in small bowel obstruction. Additionally, there was stranding of the surrounding mesentery (
Fig. 1). Radiologic findings raised suspicion of adenocarcinoma, and other etiologies such as vascular, inflammatory, or infectious process within the differential diagnosis. Laparoscopic small bowel resection was performed.
A resected segment of the small bowel was sent to pathology. It measured 12.5 cm in length and approximately 3.5 cm in average diameter. An area of narrowing was identified at 6.5 cm from one end. Opening the specimen revealed an area of constriction corresponding to the area of narrowing identified on the external surface. Sectioning through this area revealed a thickened bowel wall and an area of induration in the mesenteric fat with chalky white cut surface (
Fig. 2). Microscopic examination showed focal mucosal erosion with acute and chronic inflammation in the surrounding peri-intestinal soft and adipose tissue, granulation tissue, fat necrosis, fibrosis, hemosiderin deposition, focal foreign body giant cell reaction, and new bone formation (
Fig. 3). A diagnosis of HMO was made. On follow up, the patient reported feeling much better, having normal bowel movements, and no pain.
Case 2
A 36-year-old woman, non-smoker, presented with weakness and vomiting two months after undergoing robotic gastric sleeve resection with hiatal hernia repair due to morbid obesity, hiatal hernia, and symptomatic gastroesophageal reflux disease. The patient had multiple radiographic studies showing a stricture at the distal esophagus causing a kinking of the gastroesophageal junction. She had an esophageal stent placed across this stricture in an effort to correct the stricture/kinking. A repeat esophagogastroduodenoscopy was performed showing that the previously placed esophageal stent had migrated distally into the stomach with inability to reach the stent for repositioning. A deformed esophagogastric junction was also noted causing stenosis and deformity of the lumen, not allowing for any instrument advancement into the stomach body. An exploratory laparoscopy was performed showing an esophageal stricture due to significant abdominal scar tissue, migrated esophageal stent placed to straighten out the esophagus and significant intra-abdominal scar tissue (
Fig. 4). Consequently, she required a resection of the stricutred distal esophagus, gastroesophageal junction and stomach and her alimentary tract was reconstructed with Roux-en-Y esophagojejunostomy.
The patient had a surgical history of a breast reduction and cesarean section aside from her most recent sleeve gastrectomy procedure. She had no significant family history and no known allergies. Vital signs on admission were as follows: blood pressure 145/100 mm Hg, pulse 92 beats per minute, respiratory rate 18 per minute, temperature 36.3°C, and SpO
2 98%. BMI was 45.59 kg/m
2 (height 157.5 cm and weight 113.8 kg). Physical examination revealed a soft abdomen that is tender to deep palpation in the gastric region. Relevant laboratory results of patient 2 are summarized in
Table 2. Red cell indices and electrolyte levels were within normal limits.
A resected segment of the distal esophagus, gastroesophageal junction, stomach, and segments of small bowel were sent to pathology. The specimens were opened to reveal a tan-pink focally disrupted mucosae with rugae and areas with thickened wall measuring up to 1 cm in maximum thickness. Microscopic examination showed gastric and small intestinal mucosae with suture granulomas, foreign body giant cell reaction, and new bone formation (
Fig. 5). The diagnosis of HMO was made. On follow up, the patient reported feeling better, and tolerating oral intake without any further issues.
DISCUSSION
HMO is a rare pathologic entity with approximately 75 cases reported to date [
7,
8]. In a comprehensive review by Althaqafi al. [
7], HMO was found to occur during mid-to-late adulthood, with a predilection in the male gender (90.4%, 66/73 patients). The common complaint amongst those with HMO tends to be bowel obstruction (41.1%) or the presence of an enterocutaneous fistula (6.8%) [
7]. The diagnosis of HMO can be challenging; rarely, HMO can be diagnosed based on an incidental CT scan finding showing dense calcified shadows which aid in its preoperative identification. However, the differential diagnosis in such cases includes dystrophic calcification, bone neoplasms, leakage of contrast, foreign material, or extra-skeletal osteosarcoma [
7]. Ossification occurs in the mesentery and omentum in the majority of the diagnosed cases (89%) [
7].
The pathophysiology of HMO has not been well defined yet, but there are several mechanisms that have been postulated. The process of ectopic ossification is classified into two categories based on the histologic features: dystrophic and heterotopic [
13]. The reasons as to the involvement versus the lack of involvement of these cells remains to be investigated. Leblanc et al. [
15] found a relationship between bone morphogenic proteins (BMPs) and peripheral cellular stress pathways. Specifically, BMP-9 possessed a strong osteoinductive capacity only in damaged muscle, whereas BMP-2 promoted ossification in skeletal muscle regardless of its state [
15]. BMPs are cytokines belonging to the transforming growth factor-β family [
16]; they are released from inflammatory cells when there is stress at the site of the cellular damage. This, in turn, through a series of proliferative mechanisms, results in the formation of supplementary cartilage and bone [
15].
It is postulated that HMO forms due to the stimulation of pluripotent mesenchymal stem cells in response to inflammation from an inciting event, causing differentiation of those stem cells into osteoblasts and osteocytes, aided by osteogenic differentiation factors. In one theory, it is hypothesized that ‘seeding’ of the mesentery/omentum with activated osteoprogenitor cells during surgery can induce formation of heterotopic bone [
17]. Another theory put forward is that immature pluripotent mesenchymal cells, localized within the mesentery and omentum, differentiate into osteoblasts or chondroblasts and induce bone formation, a process called osteogenic induction [
13]. Differentiation of those cells takes place as a result of certain mechanical stimuli from previous surgery or trauma, or due to a combination of multiple stimuli. We hypothesize that there is inflammation from an inciting event (trauma/surgery) that stimulates the pluripotent mesenchymal stem cells causing their differentiation into osteoblasts and osteocytes, aided by osteogenic differentiation factors present within the mesentery (
Fig. 6). Interestingly, in one study by Kan et al. [
18], substance P, a neuropeptide responsible for the sensation of pain, was found to be dramatically increased in early lesioned tissues in patients who have either fibrodysplasia ossificans progressiva or acquired heterotopic ossification, and in three independent mouse models of heterotopic ossification. Furthermore, levels of alkaline phosphatase and calcium were found to be elevated in several cases of HMO post-surgical intervention; these markers were indicative of its recurrence [
7]. This signifies a potent neuro-inflammatory induction and amplification circuit for BMP-dependent heterotopic ossification lesion formation, identifying novel molecular targets for prevention of this condition and its recurrence [
18].
Considering all of this, physicians should not overlook this rare but significant condition and use of immunomodulators as well as thorough laboratory analysis and follow up in patients with HMO. Continuous monitoring and controlling of the inflammatory cytokines for an extended post-operative, rather than a shorter time frame, may theoretically prevent or delay the HMO formation. Although there are no recommendations at this time other than surgical intervention for treatment of HMO, further research elucidating the involvement of cytokines and potential immunomodulators must be completed given that there are promising future outcomes with these interventions. Also, theoretically, treatment should be conservative whenever possible to prevent further ossification from surgical intervention.
ACKNOWLEDGMENTS
We would like to thank all members of the Arkadi M. Rywlin M.D. Department of Pathology and Laboratory Medicine, Mount Sinai Medical Center (Miami Beach, FL, USA) for their help with this work.
References
1. Balboni TA, Gobezie R, Mamon HJ. Heterotopic ossification: pathophysiology, clinical features, and the role of radiotherapy for prophylaxis. Int J Radiat Oncol Biol Phys. 2006; 65:1289–99.

2. Naraghi FF, DeCoster TA, Moneim MS, Miller RA, Rivero D. Heterotopic ossification. Orthopedics. 1996; 19:145–51.

3. Lemeshev Y, Lahr CJ, Denton J, Kent SP, Diethelm AG. Heterotopic bone formation associated with intestinal obstruction and small bowel resection. Ala J Med Sci. 1983; 20:314–7.
4. Hansen O, Sim F, Marton PF, Gruner OP. Heterotopic ossification of the intestinal mesentery: report of a case following intraabdominal surgery. Pathol Res Pract. 1983; 176:125–30.
5. Wilson JD, Montague CJ, Salcuni P, Bordi C, Rosai J. Heterotopic mesenteric ossification (‘intraabdominal myositis ossificans’): report of five cases. Am J Surg Pathol. 1999; 23:1464–70.
6. Ferreira C, Gomes C, Melo A, et al. Heterotopic mesenteric and abdominal wall ossification: two case reports in one institution. Int J Surg Case Rep. 2017; 37:22–5.
7. Althaqafi RM, Assiri SA, Aloufi RA, Althobaiti F, Althobaiti B, Al Adwani M. A case report and literature review of heterotopic mesenteric ossification. Int J Surg Case Rep. 2021; 82:105905.

8. Sarraf K, Newman O, Mirkazemi M, Serena T. Laparoscopic enterolysis of congenital band precipitating pathogenic heterotopic mesenteric ossification requiring hemicolectomy: a case report. Am J Case Rep. 2022; 23:e934910.

9. McCarthy EF, Sundaram M. Heterotopic ossification: a review. Skeletal Radiol. 2005; 34:609–19.

10. Binesh F, Akhavan A, Navabii H, Ostadi M. Heterotopic mesenteric ossification: report of a case and review of the literature. BMJ Case Rep. 2012; 2012:bcr0220125793.

11. Gaffey MJ, Winston DC. Heterotopic ossification of soft tissue: a review with emphasis on ossification within abdominal surgical scars. Anat Pathol. 1998; 3:195–208.
12. Xu Y, Huang M, He W, et al. Heterotopic ossification: clinical features, basic researches, and mechanical stimulations. Front Cell Dev Biol. 2022; 10:770931.

13. Myers MA, Minton JP. Heterotopic ossification within the small-bowel mesentery. Arch Surg. 1989; 124:982–3.

14. Hicks CW, Velopulos CG, Sacks JM. Mesenteric calcification following abdominal stab wound. Int J Surg Case Rep. 2014; 5:476–9.

15. Leblanc E, Trensz F, Haroun S, et al. BMP-9-induced muscle heterotopic ossification requires changes to the skeletal muscle microenvironment. J Bone Miner Res. 2011; 26:1166–77.

16. Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009; 19:71–88.

17. Koolen PG, Schreinemacher MH, Peppelenbosch AG. Heterotopic ossifications in midline abdominal scars: a critical review of the literature. Eur J Vasc Endovasc Surg. 2010; 40:155–9.

18. Kan L, Lounev VY, Pignolo RJ, et al. Substance P signaling mediates BMP-dependent heterotopic ossification. J Cell Biochem. 2011; 112:2759–72.

Fig. 1
Computerized tomography scan of the abdomen and pelvis with intravenous contrast. Results showed segmental concentric thickening of the jejunum in the right upper quadrant of the abdomen (with apple core configuration, white arrowhead) resulting in small bowel obstruction and stranding of the surrounding mesentery.
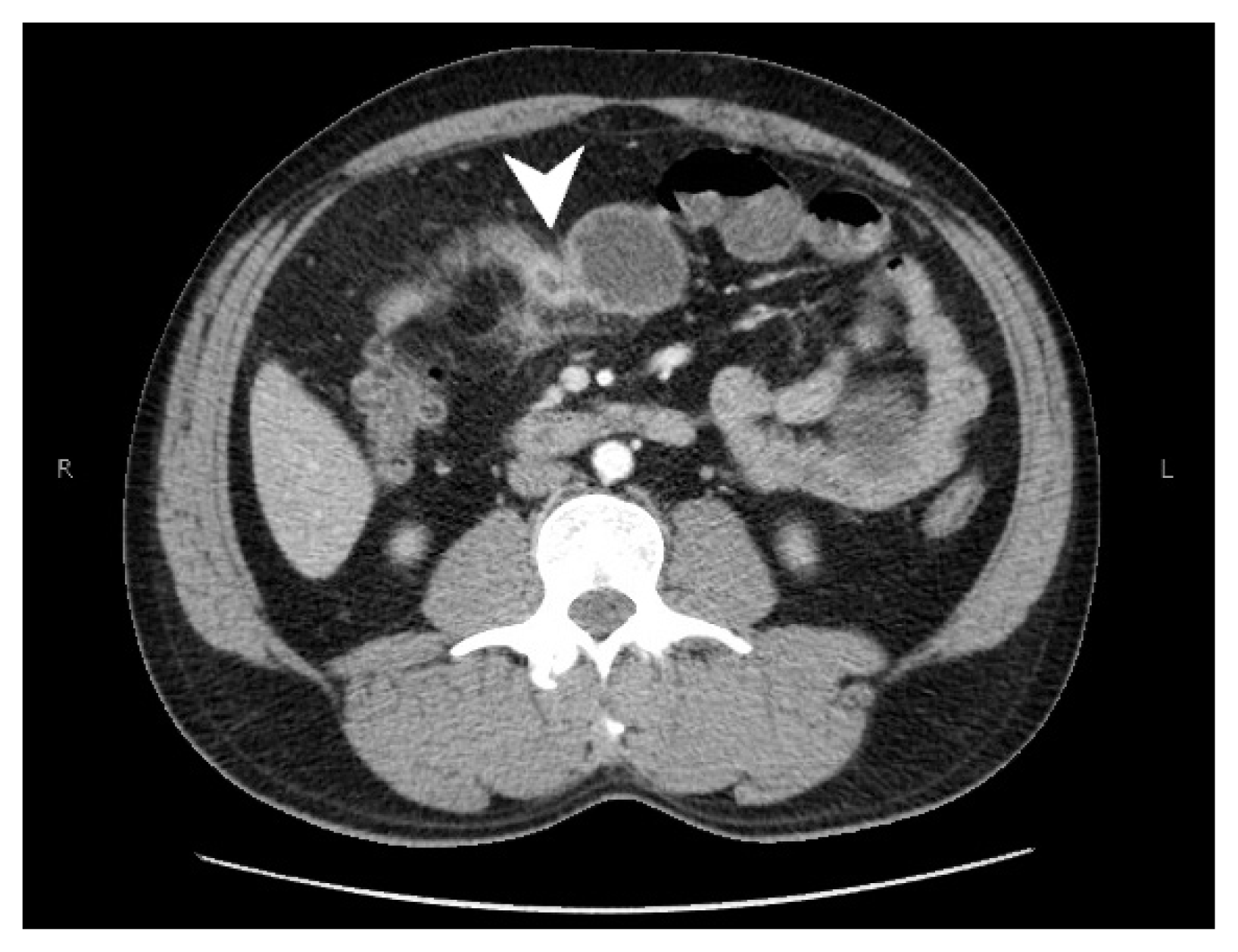
Fig. 2
Gross examination of the resected segment of small intestine. (A) An area of luminal constriction is seen. (B) Induration in the surrounding mesenteric fat showing chalky white cut surface.
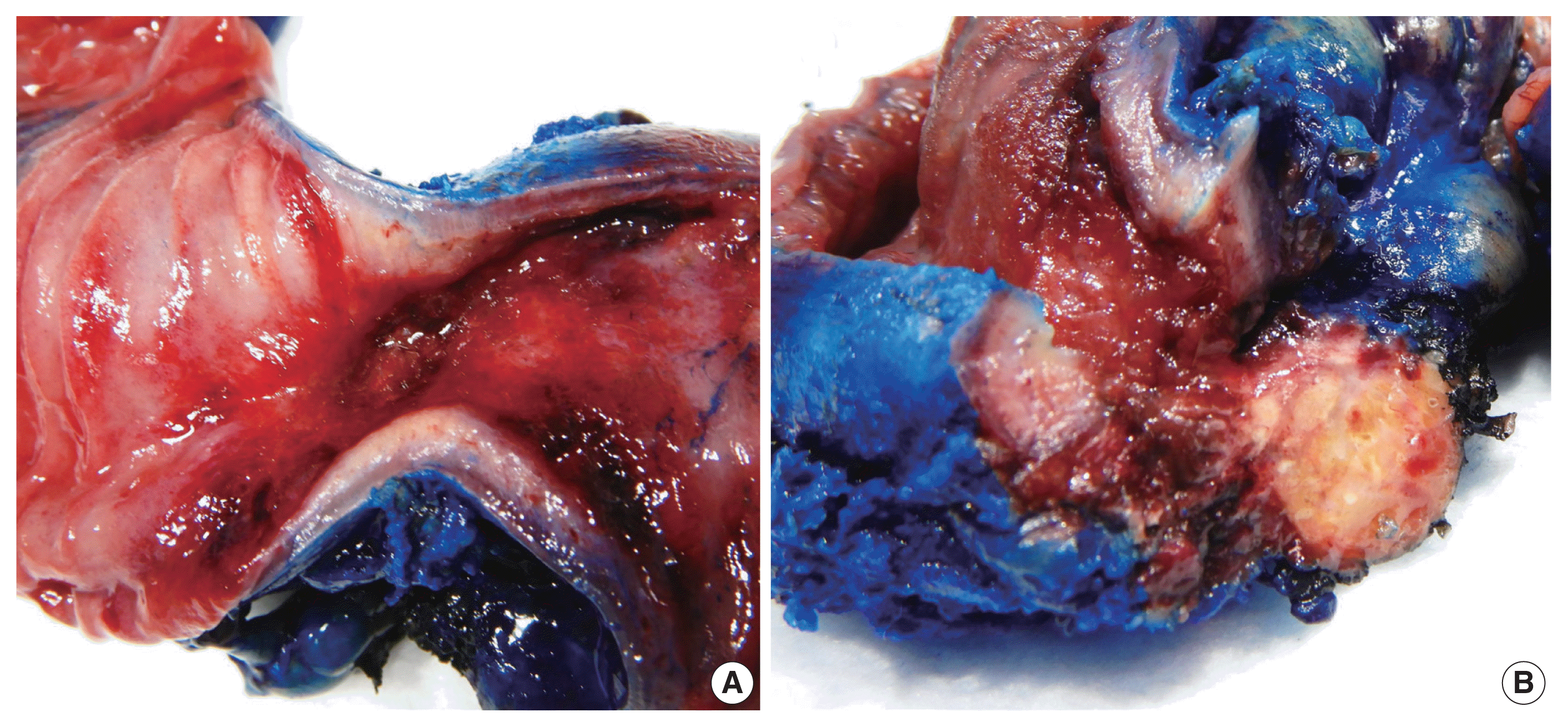
Fig. 3
Microscopic examination showing heterotopic ossification. Histopathologic examination of the resected segment of small intestine demonstrated focal mucosal erosion (A) with acute and chronic inflammation in the surrounding peri-intestinal soft and adipose tissue, granulation tissue, fat necrosis (B), fibrosis, hemosiderin deposition, focal foreign body giant cell reaction (C), and new bone formation (D).

Fig. 4
Exploratory laparoscopy showing significant abdominal scar tissue and previous sleeve gastrectomy.
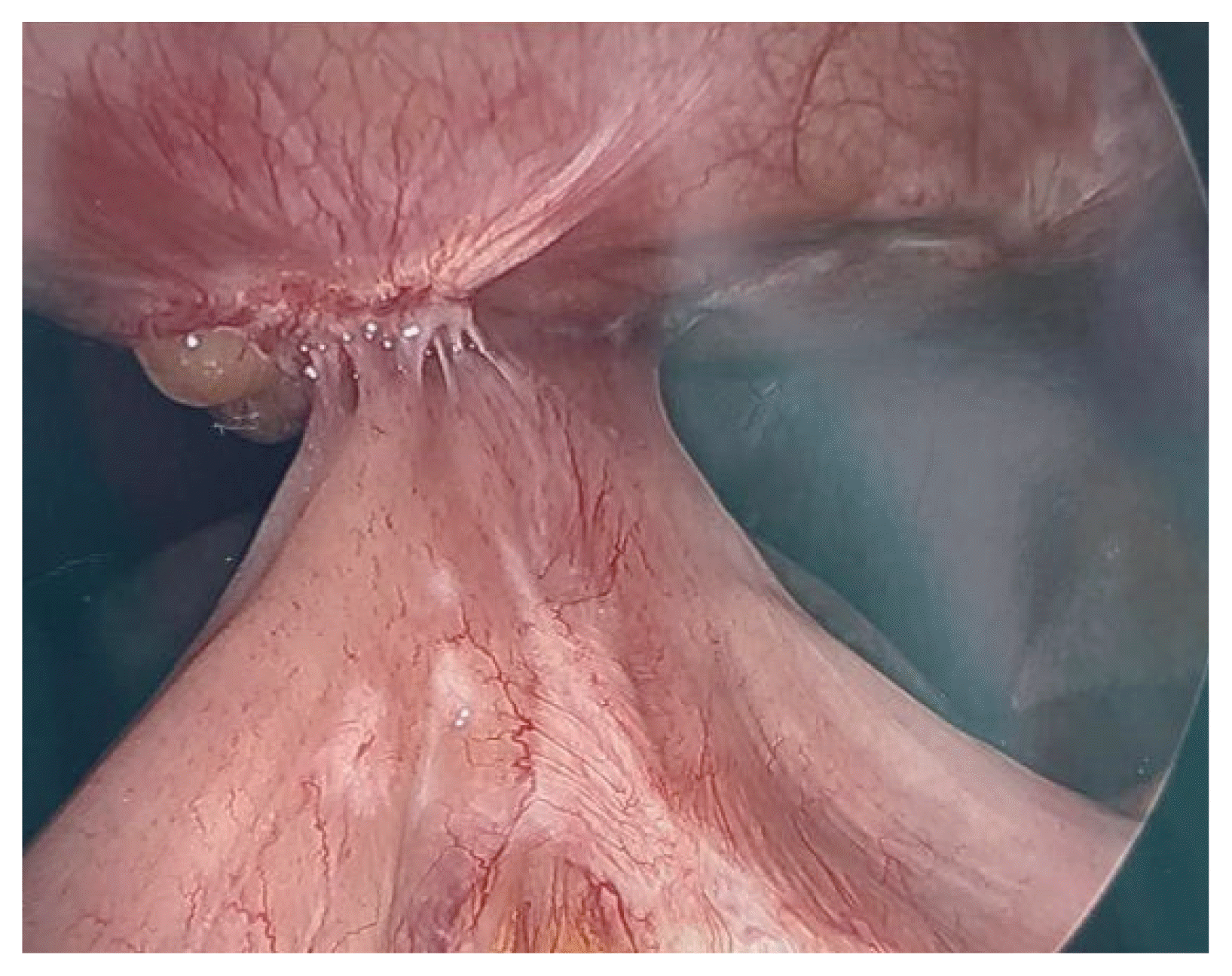
Fig. 5
Microscopic examination showing heterotopic ossification. Histopathologic examination of the resected segments of stomach and small intestine demonstrated chronic inflammation, suture granulomas, foreign body giant cell reaction (A), and new bone formation (B).
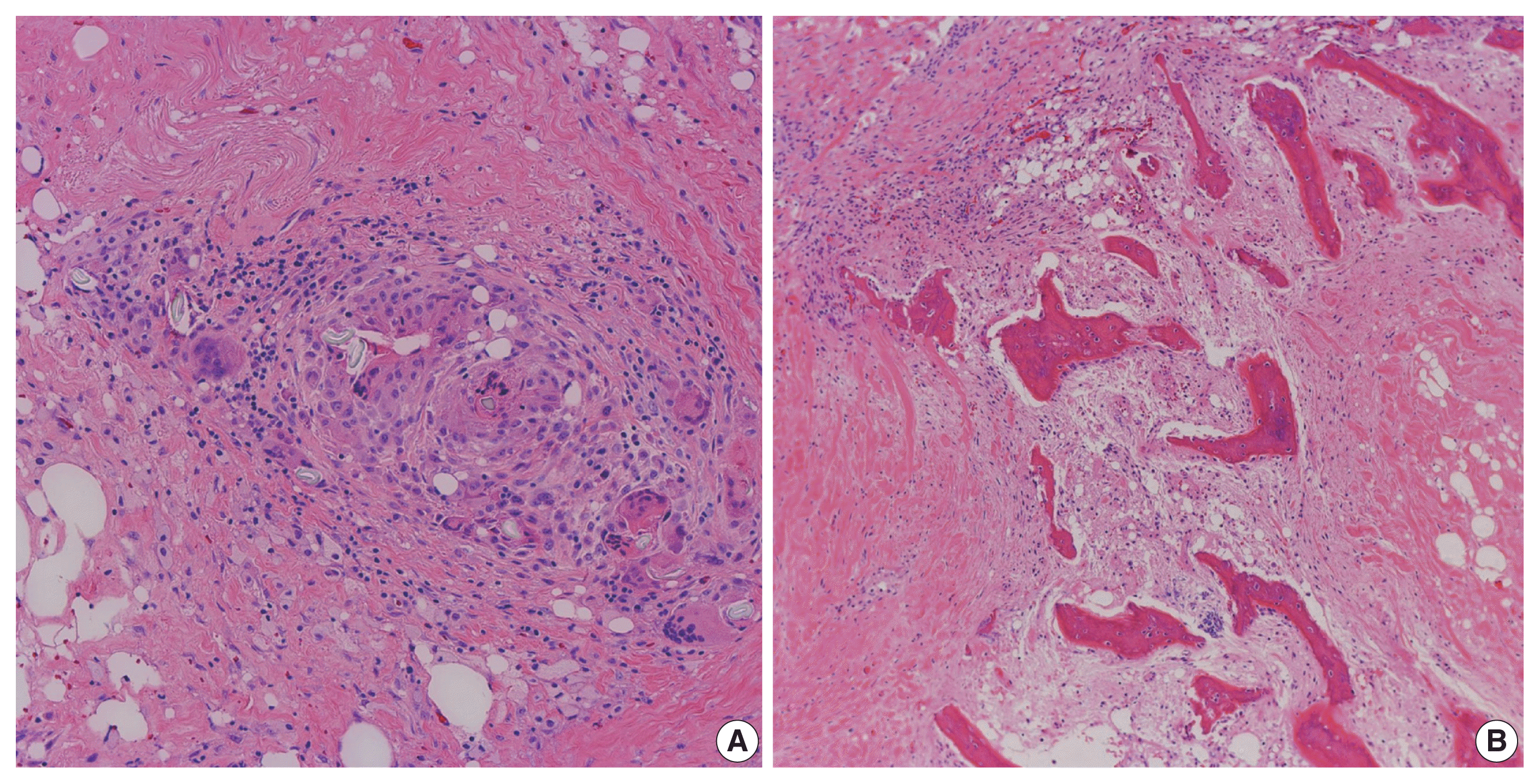
Fig. 6
Schematic diagram illustrating the proposed hypotheses for the formation of heterotopic mesenteric ossification. ECM, extracellular matrix; ALP, alkaline phosphatase; BMP, bone morphogenetic protein; TGF-β, transforming growth factor-β; PTH, parathyroid hormone; IGH-1, insulin-like growth factor 1; FGF, fibroblast growth factor.
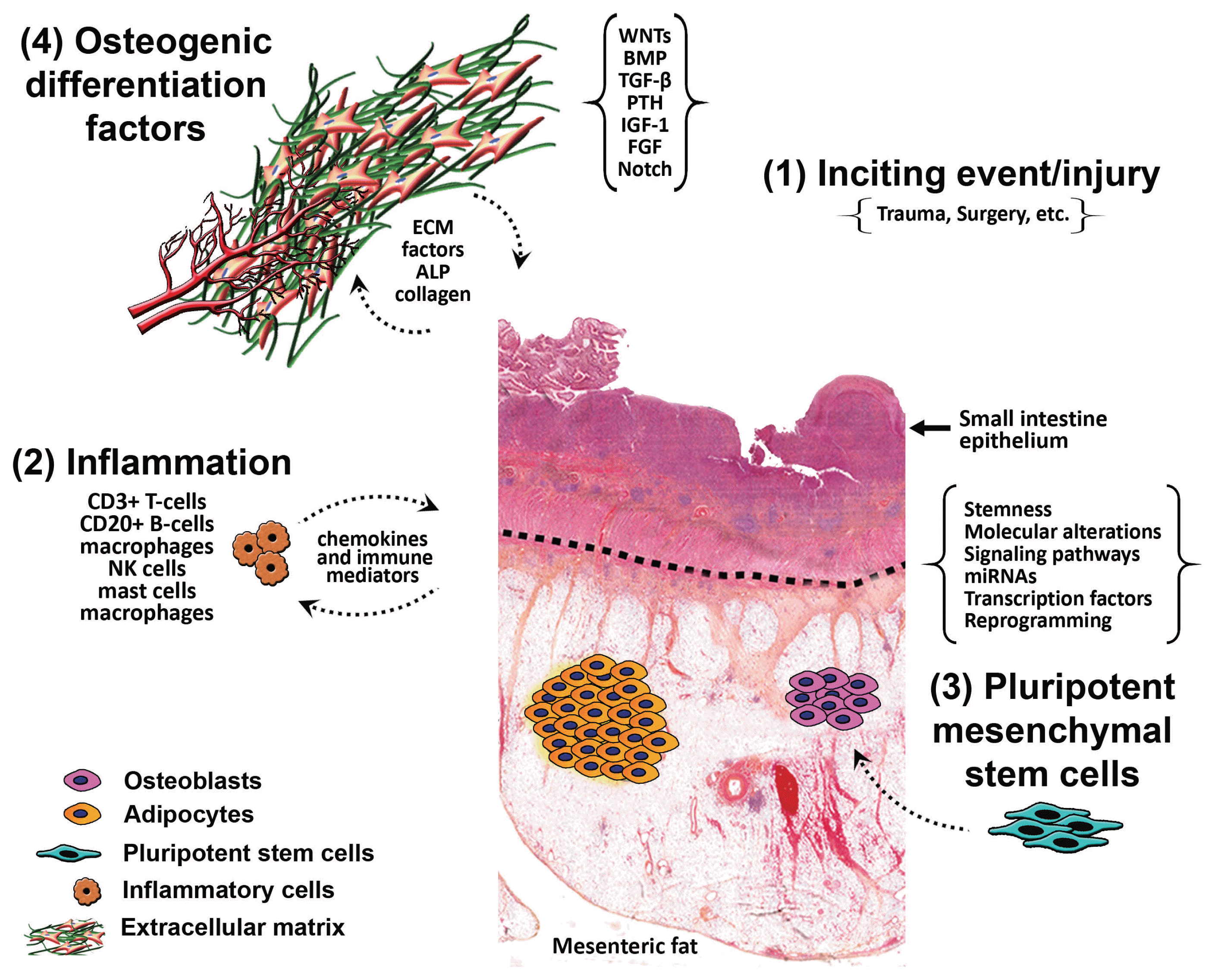
Table 1
Relevant laboratory results of patient 1 on admission
|
Patient laboratory value |
Reference range |
|
White blood cell count (× 103/μL) |
13.47 |
4.8–10.8 |
|
Segmented neutrophils (%) |
80.7 |
42–75 |
|
Absolute neutrophil count (× 103/μL) |
10.87 |
1.8–7.2 |
|
Lymphocytes (%) |
12.3 |
16.0–45.0 |
|
Red blood cell count (× 106/μL) |
5.08 |
3.93–5.22 |
|
Hemoglobin (g/dL) |
15.0 |
12.0–16.0 |
|
Hematocrit (%) |
45.7 |
37.0–47.0 |
|
Serum creatinine (mg/dL) |
1.06 |
0.55–1.02 |
|
Blood urea nitrogen (mg/dL) |
20.0 |
7–18 |
|
Alkaline phosphatase level (U/L) |
141 |
46–116 |
|
Lactate level (mmol/L) |
0.4 |
0.4–2.0 |
Table 2
Relevant laboratory results of patient 2 on admission
|
Patient laboratory value |
Reference range |
|
White blood cell count (× 103/μL) |
8.47 |
4.8–10.8 |
|
Red blood cell count (× 106/μL) |
4.43 |
3.93–5.22 |
|
Hemoglobin (g/dL) |
14.7 |
12.0–16.0 |
|
Hematocrit (%) |
45.6 |
37.0–47.0 |
|
Serum creatinine (mg/dL) |
1.21 |
0.55–1.02 |
|
Blood urea nitrogen (mg/dL) |
18.0 |
7–18 |










 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download