Abstract
Objective
To demonstrate the sonoanatomy of the medial antebrachial cutaneous nerve (MACN) in the elbow region using high-resolution ultrasonography (HRUS) to identify areas at a high risk of MACN injury.
Methods
A total of 44 arms were included in the study. In the supine position, the participants’ arms were abducted 45° with the elbow fully extended. The MACN was visualized in the transverse view. The anterior branch of the MACN (ABMACN), posterior branch of the MACN (PBMACN), and location of the branching sites were determined. The distance between the ABMACN and superficial veins, including the basilic vein (BV) and median cubital veins (MCV) was measured. For the PBMACN, the distance to the ulnar nerve (UN) and to BV were measured.
Results
The MACN was subdivided into 2.18±1.00 branches, including ABMACN and PBMACN. The ABMACN and PBMACN were subdivided into 1.60±0.78 and 1.07±0.25 branches, respectively. The branching point of the MACN was 8.40±2.42 cm proximal to the interepicondylar line (IEL). We demonstrated that the ABMACN is located close to the BV and MCV in the elbow region, and the PBMACN was located approximately 1 cm and 0.8 cm anterior to the UN and posterior to the BV at the IEL level, respectively.
The medial antebrachial cutaneous nerve (MACN) is a pure sensory nerve that innervates the distal anteromedial arm, antecubital fossa, posterior olecranon, and anteromedial forearm [1]. The MACN arises from the medial cord or lower trunk of the brachial plexus and mainly consists of C8 and T1 nerve root fibers [1–3]. In the axilla, the MACN is located atop the axillary artery and vein, which is near the median and ulnar (UN) nerves [4]. In the arm, the MACN courses between the brachialis and triceps brachii muscles, and becomes subcutaneous at the distal or mid-brachial level running close to the basilic vein (BV) [1,3–5]. Between the mid- and lower thirds of the arm, the MACN passes the basilic hiatus with the BV and commonly divides into the anterior branch (ABMACN) and posterior branch (PBMACN) [1,2,6–8]. After piercing the brachial fascia at the elbow, the ABMACN anteriorly crosses the elbow between the biceps tendon and medial epicondyle (ME) [1,9]. Meanwhile, the PBMACN runs medial to the BV and crosses the elbow posteriorly toward the medioposterior aspect of the forearm [1,5,8]. Reportedly, the distance of the PBMACN from the ME varies and the PBMACN can be located 6 cm proximal or 4 cm distal to the ME [1].
MACN can be injured due to various iatrogenic invasive procedures around the elbow. ABMACN injuries may occur during venous puncture (such as that of the BV or median cubital vein [MCV]) [10–12]. PBMACN is at risk of damage in cubital tunnel surgery [11,13–16], elbow arthroscopy [17,18], open elbow surgery [19], and steroid injection for medial epicondylitis [20]. A study has also reported on the risk to PBMACN during routine venous puncture [12]. Therefore, identifying the location of the MACN, including all distal branches, such as the ABMACN and PBMACN, around the elbow is necessary for avoiding injury during a procedure.
Limited ultrasonographic studies have reported about MACN [4,8,11,21]. Moreover, in these studies, the course of the MACN branches could not be described in detail because they were not traced as distally as the elbow, where the risk of injury was high. Specifically, no previous study has observed the PBMACN using ultrasonography. Therefore, in this study, we have described the anatomical location of the MACN, including its branches (ABMACN and PBMACN), using ultrasonography; further, we have suggested a high-risk zone susceptible to MACN injury around the elbow.
Healthy adult volunteers were prospectively recruited from February 2021 to February 2022. Volunteers with a history of cervical radiculopathy, peripheral neuropathy, upper extremity trauma, or medical conditions associated with peripheral polyneuropathy were excluded. Baseline data such as age, sex, height, weight, arm length (distance from the acromion to the interepicondylar line [IEL]), and IEL length were collected.
Each participant was informed of the study protocols and all provided informed consent. This study was approved by the Institutional Review Board of Korea University Guro Hospital (IRB No. 2020GR0360).
The arms were scanned using high-resolution ultrasonography (HRUS) (RS80A; Samsung Medison, Seoul, Korea) with a 3–16 MHz linear array transducer. A rehabilitation physician performed all ultrasonographic examinations (Kim JM).
The participants were examined in the supine position, with the arm abducted at 45° and elbow fully extended. The MACN was visualized in short-axis view. After identification of the MACN at the axillary level, it was traced distal to the IEL. Each branch (ABMACN and PBMACN) was identified, and the location of the branching site was determined. For tracing the ABMACN and PBMACN, the probes were placed parallel and perpendicular to the IEL, respectively. We quantified the positional relationship between the MACN and major structures at the level of the IEL and 4 cm proximal to the IEL (IEL+4 cm).
Furthermore, we identified the location of the ABMACN at the IEL level, distance from the BV and MCV to the ABMACN at the IEL level, and distance from the UN or BV to the PBMACN at the IEL and IEL+4 cm levels. Fig. 1 illustrates each representative ultrasonographic view used for the measurement. When measuring the distance between the structures, the shortest distance from the closest edge point was measured along the mediolateral and anteroposterior axes.
All statistical analyses were performed using IBM SPSS Statistics version 26 (IBM Corp., Armonk, NY, USA). Data normality was assessed using the Kolmogorov-Smirnov and Shapiro-Wilk tests. Depending on whether the data were normally distributed, quantitative data were presented as mean±standard deviation or median with interquartile range (IQR). Qualitative data were reported as frequencies and percentages. Statistical significance was set at p<0.05.
In total, 44 arms of 22 healthy adults were included in the study. The general characteristics of the participants are listed in Table 1. On the transverse scan, the MACN appeared as a rounded, hyperechoic fascicle. At the axillary and proximal arm levels, the MACN ran distally with the BV, axillary or brachial artery, median nerve, and UN. At the mid-arm level, the MACN was located near the BV and branched into the ABMACN and PBMACN. At the distal arm level, the ABMACN was located on the anteromedial elbow, while the PBMACN coursed toward the ME or UN.
Despite observing the MACN from the axilla to mid-arm level in all participants using the HRUS, tracing it to the IEL level was difficult in 3 of the 44 arms because the diameter of the MACN became too small to be detected. The ABMACN and PBMACN were detected distal to the IEL level in 30 of the 44 arms. In four arms, tracing the ABMACN to the IEL level was not possible. In 14 arms, the PBMACN could not be traced to the IEL level. The average number of MACN branches was 2.18±1.00; the ABMACN had 1.60±0.78 branches, whereas the PBMACN had 1.07±0.25 branches. The MACN branched into ABMACN and PBMACN at a mean distance of 8.40±2.42 cm proximal to the IEL.
The positional relationship between the ABMACN and its major structures is summarized in Table 2. The ABMACN was located near the BV at the IEL+4 cm level. At the IEL level, it was located near the BV or MCV.
The positional relationship between the PBMACN and the major structures is described in Table 3. The PBMACN was located at a median distance of 1.11 cm (IQR, 0.4–1.8 cm) anterior to the UN, which is generally located just posterior to the ME in the elbow region.
We observed the MACN and its branches (ABMACN and PBMACN) in the elbow region of healthy volunteers using HRUS. To the best of our knowledge, only a few original studies have focused on MACN using ultrasound [8,21]. However, in these previous studies, the main anatomical structure of interest differed from those in our study because they did not focus on invasive procedures in the elbow region. Thallaj et al. [8] focused on identifying the MACN at the mid-arm level for ultrasound-guided MACN blockade. Oh et al. [21] focused on identifying the ideal electrical stimulation site of the MACN in the distal arm, specifically the distance between the MACN and median nerve. Although two other studies reviewed methods of observing MACN by ultrasonography [4,11], these mainly qualitatively described the examination methods at the arm level. Thus, this study is the first ultrasound study to quantitatively investigate the positional relationship between MACN branches and major landmarks in the elbow region, where MACN injury frequently occurs.
As previous cadaveric studies have demonstrated, the course and number of MACN branches are highly variable [1,3,5–7]. Tanaka and Lourie [5] suggested that the PBMACN can be subdivided into 1–4 branches (mean, 2.07). Li et al. [6] demonstrated that the MACN was divided into two primary nerve branches (anterior and posterior) in 23 of 24 specimens, whereas single trunk was observed in one specimen. They also suggested that the ABMACN projected into 7–10 branches, whereas the PBMACN projected into 10–12 secondary branches. Race and Saldana [3] suggested that the MACN was distributed into 1–3 branches anteriorly innervating the proximal medial forearm, whereas 3–7 branches passed posteriorly, close to the cubital tunnel. In a cadaveric study by Manoukov et al. [7], the PBMACN was detected in all cases, with an average of 1.32 secondary branches. In our study, the average number of MACN branches was 2.18±1.00, the number of secondary branches of the ABMACN was 1.60±0.78, and that of the secondary branches of the PBMACN was 1.07±0.25. Fewer MACN branches were detected in our study than in previous cadaveric studies. This is because relative to the observation with naked eye during direct dissection, observing the distal branches of the MACN with HRUS is more difficult because it becomes too thin to be detected.
Masear et al. [1] demonstrated that the ABMACN and PBMACN become distinct at a mean distance of 14.5 cm proximal to the ME. Race and Saldana [3] reported that the MACN gave branches at a distance of 4–6 cm proximal to the ME in 60% of the specimens and further split into 5–8 branches at 2 cm proximal to the ME. Tanaka and Lourie [5] suggested that the MACN was subdivided at a mean distance of 6.35 cm proximal to the ME. Sin et al. [22] dissected 29 upper extremities and demonstrated that the MACN divided into two branches at 10.83±6.27 cm proximal to the ME. Consistent with these previous studies, in our study, the MACN subdivided at a mean distance of 8.40±2.42 cm proximal to the IEL. Therefore, the examination and procedure should be performed keeping in mind that the branching of the MACN starts from the mid-arm level.
Sin et al. [22] suggested that the MACN is located 2.86±0.69 cm laterally from the ME at the IEL level. Similarly, our study demonstrated that the horizontal distance between the ABMACN and the ME at the IEL level was 2.35 cm (IQR, 1.50–3.45 cm). Considering this, the location of the ABMACN can be estimated using the distance from the ME. This may be useful in patients whose BV or MCV, which were demonstrated to be located near the ABMACN (Table 2) are not visible to the skin with the naked eye. Therefore, this finding can help lower the risk of ABMACN injury during procedures such as vein sampling around the elbow region.
In a cadaveric study, Manoukov et al. [7] investigated the relationship between the PBMACN and other major anatomical landmarks. However, their measurement method was different from that used in this study. First, the authors focused on PBMACN injury during cubital tunnel surgery and studied cadavers in the elbow flexed position (at 45° and 90°). Second, they measured the distance of interest at the forearm level using a line extending from the ME to the distal radioulnar joint. They measured two distances: (1) between the PBMACN and ME and (2) between the PBMACN and UN. However, since our study was an ultrasonographic study and not a cadaveric study, it was difficult to trace the MACN down to the forearm level, so we only observed the MACN branches as distal as the IEL level. In addition, since both the ABMACN and PBMACN had to be localized using nearby landmarks (ME, BV, etc.), the participants’ elbow was fully extended to ensure the consistency of this process.
This study has some limitations. Considering that the MACN has frequent anatomical variations, our sample size may have been insufficient, although it was larger than that in previous ultrasonographic studies. Therefore, further studies should include a larger sample size. In our study, the ultrasound examination was performed only when the elbow was fully extended. If the examination is performed with the elbow in a flexed position, subluxation or dislocation of the ulnar nerve could occur; however, this was not considered in our study. Manoukov et al. [7] suggested that when the elbow was flexed 90°, the incision site in cubital tunnel release surgery was distant from the PBMACN. However, when the elbow was flexed 45°, the incision site crossed the MACN location, increasing the risk of injury. Therefore, in future studies, dynamic values based on the degree of elbow flexion should be measured for the PBMACN to obtain more clinically practical and valuable results, as cubital tunnel surgery or injection is usually performed with the elbow flexed.
In conclusion, our study is the first to demonstrate that the course of the MACN can be traced distally to the interepicondylar level. The relative locations of the ABMACN and PBMACN with the adjacent BV, UN, and ME were identified using HRUS. Based on our findings, clinicians can perform invasive procedures around the elbow region more carefully and lower the risk of MACN injury.
ACKNOWLEDGMENTS
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (No. NRF-2020R1A2C1009024).
Notes
Conceptualization: Kim JM, Yoon JS. Methodology: Kim JM, Yoon JS. Formal analysis: all authors. Funding acquisition: Yoon JS. Project administration: Yoon JS. Visualization: Kim JM. Writing – original draft: Kim JM. Writing – review and editing: all authors. Approval of final manuscript: all authors.
REFERENCES
1. Masear VR, Meyer RD, Pichora DR. Surgical anatomy of the medial antebrachial cutaneous nerve. J Hand Surg Am. 1989; 14(2 Pt 1):267–71.

2. Hollinshead WH. Arm, elbow, and forearm. Anat Surg. 1964; 3:400–23.
3. Race CM, Saldana MJ. Anatomic course of the medial cutaneous nerves of the arm. J Hand Surg Am. 1991; 16:48–52.

4. Chang KV, Mezian K, Nanka O, Wu WT, Lou YM, Wang JC, et al. Ultrasound imaging for the cutaneous nerves of the extremities and relevant entrapment syndromes: from anatomy to clinical implications. J Clin Med. 2018; 7:457.

5. Tanaka SK, Lourie GM. Anatomic course of the medial antebrachial cutaneous nerve: a cadaveric study with proposed clinical application in failed cubital tunnel release. J Hand Surg Eur Vol. 2015; 40:210–2.

6. Li H, Zhu W, Wu S, Wei Z, Yang S. Anatomical analysis of antebrachial cutaneous nerve distribution pattern and its clinical implications for sensory reconstruction. PLoS One. 2019; 14:e0222335.

7. Manoukov Y, Herisson O, Sali E, Sautet A, Masquelet AC, Cambon-Binder A. Anatomy of the posterior branch of the medial antebrachial cutaneous nerve: a cadaveric study. Orthop Traumatol Surg Res. 2020; 106:771–4.

8. Thallaj A, Marhofer P, Kettner SC, Al-Majed M, Al-Ahaideb A, Moriggl B. High-resolution ultrasound accurately identifies the medial antebrachial cutaneous nerve at the midarm level: a clinical anatomic study. Reg Anesth Pain Med. 2011; 36:499–501.

9. Damwan A, Agthong S, Amarase C, Yotnuengnit P, Huanmanop T, Chentanez V. Medial antebrachial cutaneous nerve: anatomical relationship with the medial epicondyle, basilic vein and brachial artery. Int J Morphol. 2014; 32:481–7.

10. Kim HJ, Park SK, Park SH. Upper limb nerve injuries caused by intramuscular injection or routine venipuncture. Anesth Pain Med. 2017; 12:103–10.

11. Bianchi S, Becciolini M, Urigo C. Ultrasound imaging of disorders of small nerves of the extremities: less recognized locations. J Ultrasound Med. 2019; 38:2821–42.

12. Horowitz SH. Peripheral nerve injury and causalgia secondary to routine venipuncture. Neurology. 1994; 44:962–4.

13. Sarris I, Gobel F, Gainer M, Vardakas DG, Vogt MT, Sotereanos DG. Medial brachial and antebrachial cutaneous nerve injuries: effect on outcome in revision cubital tunnel surgery. J Reconstr Microsurg. 2002; 18:665–70.

14. Chen J, Tan J, Zhu B, Tang JB. How to avoid damaging the terminal branches of the medial antebrachial cutaneous nerve and the medial brachial cutaneous nerve during operations involving the medial elbow? Hand. 2016; 11(1 suppl):48S–49S.

15. Dellon AL, MacKinnon SE. Injury to the medial antebrachial cutaneous nerve during cubital tunnel surgery. J Hand Surg Br. 1985; 10:33–6.

16. Mackinnon S, Dellon A. Ulnar nerve entrapment at the elbow. Surgery of the peripheral nerve. Stuttgart, Germany: Georg Thieme Verlag;1988. p. 217–73.
17. Kelly EW, Morrey BF, O’Driscoll SW. Complications of elbow arthroscopy. J Bone Joint Surg Am. 2001; 83:25–34.

18. Claessen FM, Kachooei AR, Kolovich GP, Buijze GA, Oh LS, van den Bekerom MP, et al. Portal placement in elbow arthroscopy by novice surgeons: cadaver study. Knee Surg Sports Traumatol Arthrosc. 2017; 25:2247–54.

19. Yildiz N, Ardic F. A rare cause of forearm pain: anterior branch of the medial antebrachial cutaneous nerve injury: a case report. J Brachial Plex Peripher Nerve Inj. 2008; 3:10.

20. Richards RR, Regan WD. Medial epicondylitis caused by injury to the medial antebrachial cutaneous nerve: a case report. Can J Surg. 1989; 32:366–7. 369
21. Oh CH, Park NS, Kim JM, Kim MW. Determination of an ideal stimulation site of the medial antebrachial cutaneous nerve using ultrasound and investigation of the efficiency. Ann Rehabil Med. 2014; 38:836–42.

22. Sin JY, Kim DH, Bun HR, Hwang MR, Kang YK, Kwon HK, et al. Anatomical considerations of lateral and medial antebrachial cutaneous nerves. J Korean Acad Rehabil Med. 2007; 31:329–32.
Fig. 1
Ultrasound image of (A) the ABMACN at the IEL+4 cm level, (B) the ABMACN at the IEL level, (C) the PBMACN at the IEL+4 cm level, and (D) the PBMACN at the IEL level. ABMACN, anterior branch of the medial antebrachial cutaneous nerve; BV, basilic vein; IEL, interepicondylar line; IEL+4 cm, 4 cm proximal to the interepicondylar line; ME, medial epicondyle; MN, median nerve; PBMACN, posterior branch of the medial antebrachial cutaneous nerve; MCV, median cubital vein; TB, triceps brachii; UN, ulnar nerve.
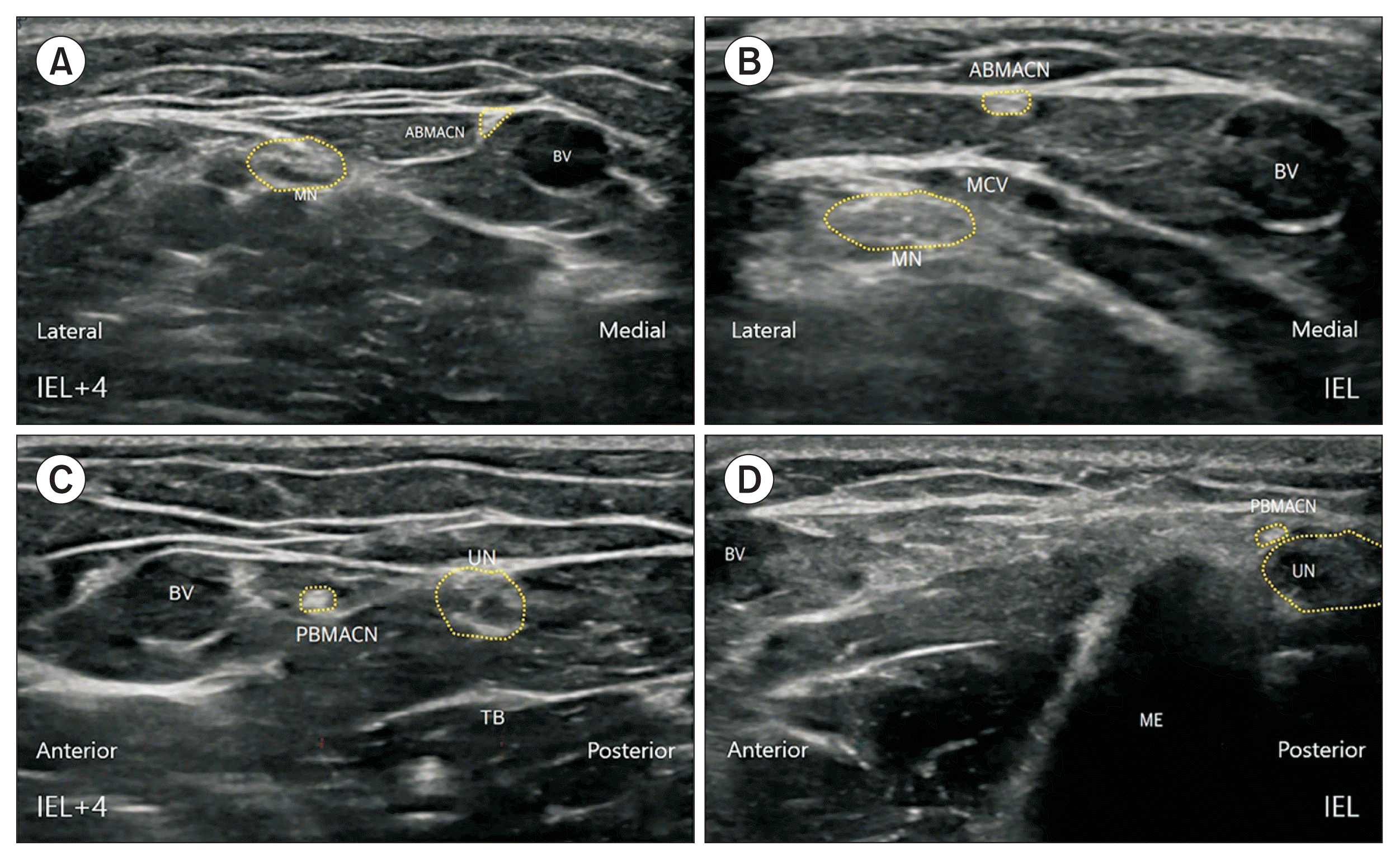
Table 1
General characteristics of the healthy volunteers (n=22, 44 arms)
Table 2
Positional relationship between the ABMACN and major structures (n=40 arms)a)
Table 3
Positional relationship between the PBMACN and major structures (n=30 arms)a)




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download