Abstract
Cerebral embolic stroke caused by a thrombus in the pulmonary vein stump after left pulmonary lobectomy is a serious complication. We retrospectively analyzed four patients who underwent mechanical thrombectomy for large-vessel occlusion after left pulmonary lobectomy between January 2014 and March 2022. Two cases occurred after left upper lobectomy and the others occurred after left lower lobectomy. All patients presented with cerebral embolic stroke from the day after surgery to the 9th postoperative day, and successful reperfusion was achieved in all cases. Two patients had good outcomes at 90 days. Thrombus in the pulmonary vein stump is the probable cause of cerebral embolism, and mechanical thrombectomy is effective. Further studies are required to establish preventive measures and perioperative management strategies.
The incidence of acute cerebral embolism in the perioperative period after pulmonary lobectomy is approximately 0.4–0.6% [1,2]. A thrombus in the pulmonary vein stump (PVS) is considered to cause cerebral embolism, which can result in serious complications [3-6]. In this case series, we report four cases who underwent mechanical thrombectomy (MT) for cerebral embolism due to the thrombus in the PVS following left pulmonary lobectomy, with a discussion of the literature.
This study retrospectively analyzed patients who underwent MT for large-vessel occlusion after left pulmonary lobectomy at our hospital between January 2014 and March 2022. The diagnosis and treatment indications for MT were determined using magnetic resonance imaging at our hospital. We analyzed age, sex, imaging findings, endovascular treatment details, and angiographic and clinical outcomes from the medical records. The time from onset to puncture (O2P), time from puncture to reperfusion (P2R), treatment device, and angiographic reperfusion were examined. The degree of reperfusion was defined using the thrombolysis in cerebral infarction (TICI) classification. TICI2b was defined as reperfusion of at least 50% of the occluded vessel-dominant territory and TICI3 was defined as complete reperfusion. The modified Rankin Scale (mRS) at 90 days was used to evaluate clinical outcomes, with an mRS of 0–2 defined as a good outcome.
In our department, a 9Fr sheath is usually placed in the right femoral artery, and we navigated with a 9Fr balloon guiding catheter (usually 9Fr Optimo; Tokai Medical Products, Aichi, Japan) and a 6Fr JB2 type coaxial catheter. Marksman (Medtronic, Minneapolis, MN, USA) was used for the microcatheter, and a 0.014-inch CHIKAI guidewire (Asahi Intecc Co., Aichi, Japan) was used for the microwire. Device selection, including the stent retriever and aspiration catheter, was determined based on the vascular anatomy of the patient and the decision of the operator.
This study included four patients, and their baseline characteristics are summarized in Table 1. The median age was 73 years, and all patients were female. The median National Institutes of Health Stroke Scale (NIHSS) score was 15 and the median Diffusion-Weighted Imaging-Alberta Stroke Program Early Computed Tomography Score (DWI-ASPECTS) was 7. All four patients were postoperative for left lung cancer, including two patients who underwent left-sided upper lobectomy (LUL) and two patients who underwent left-sided lower lobectomy (LLL). All patients presented with cerebral embolism from the day after surgery to postoperative day (POD) 9, and recombinant tissue plasminogen activator (rt-PA) was not applicable. Three patients had middle cerebral artery (MCA) occlusion and one had internal carotid artery (ICA) occlusion. Case 4 involved a patient who was transferred from another hospital. All four patients underwent MT with successful reperfusion; the median O2P was 171 minutes and the median P2R was 35 minutes. Two patients had a good outcome with mRS ≤2 at 90 days.
We describe two representative cases in detail.
An elderly patient underwent a LUL for lung cancer. On POD 6, the patient presented with disturbance of consciousness and paralysis of the right upper and lower limbs. The NIHSS score was 16, and DWI-ASEPCTS score was 9 (Fig. 1A, B). Emergency magnetic resonance angiography (MRA) revealed left ICA occlusion (Fig. 1C). Digital subtraction angiography (DSA) revealed left ICA occlusion (Fig. 1D). After 2 passes of Penumbra ACE68 aspiration catheter (Penumbra, Alameda, CA, USA), TICI3 reperfusion was performed (Fig. 1E). The thrombus was characterized as a red fibrin thrombus. A chest contrast-enhanced computed tomography (CT) scan suggested a residual thrombus in the left PVS (Fig. 1F). The patient was treated with oral anticoagulants (apixaban) and discharged to her home. The mRS was 0 after 90 days.
An elderly patient underwent video-assisted thoracoscopic surgery for LUL for lung cancer. On POD 9, the patient presented with disturbance of consciousness and right conjugate deviation. The NIHSS score was 14 and DWI-ASPECTS score was 7 (Fig. 2A). MRA revealed right MCA occlusion (Fig. 2B). We performed emergency MT and DSA revealed right M1 occlusion (Fig. 2C). TICI3 reperfusion was obtained with a single pass using Catalyst 6 (Stryker, Kalamazoo, MI, USA) (Fig. 2D). A chest contrast-enhanced CT scan showed no thrombus in the left PVS (Fig. 2E). The patient was treated with oral anticoagulants (rivaroxaban) and transferred to a recovery rehabilitation hospital on POD 40. The mRS was 3 after 90 days.
Herein, we report four patients who underwent MT for cerebral embolism after pulmonary lobectomy. The causes of cerebral embolism in the perioperative period after pulmonary lobectomy are air embolism, paradoxical embolism, atrial fibrillation, and thrombus in the PVS [4-6]. It has been reported that atrial fibrillation occurs at a rate of 10–30% after pulmonary lobectomy; however, no paroxysmal atrial fibrillation was detected with Holter electrocardiography in any of the four patients in this study [7,8]. No other stroke risk factors (diabetes mellitus, congestive heart failure, or carotid artery stenosis) were identified. We considered that these cases were likely to be cerebral embolism due to thrombus in the PVS, judging from the clinical course and post-left pulmonary lobectomy.
Ohtaka et al. [4] reported a thrombus in the left PVS in seven of 193 patients (3.6%) after pulmonary lobectomy, and all seven patients had received LUL. It has been suggested that thrombus formation is related to the PVS length [9]. To prevent congestion of blood flow in the PVS, the length of the PVS can be shortened, but this is associated with the risk of serious complications, such as hemorrhage and cardiac tamponade [10,11]. The Japanese Society of Pulmonary Surgeons revealed that thrombi in the PVS develop in the early postoperative period and are significantly more frequent in left-sided surgery (particularly after LUL). The odds ratio of cerebral infarction within 90 days for LUL to LLL was 2.07, suggesting that LUL is also involved in cerebral infarction [12]. In our series, two cases occurred after LUL, and the other two cases occurred after LLL. Usui et al. [13] reported a case of cerebral embolism caused by a thrombus in the PVS after LLL.
In this series, thrombus in the PVS was suspected on imaging in only one case. In contrast, a previous report did not show evidence of thrombus in the PVS in any case [14,15]. Xie et al. [14] reported thrombosis of the PVS in five of eight stroke patients who underwent contrast-enhanced chest CT, while three patients had no thrombosis in the PVS. Morinaga et al. [15] also excluded other embolic sources and concluded that PVS thrombus caused cerebral embolism, although they did not perform contrast-enhanced CT. Treatment (including the prevention of recurrence) is generally anticoagulation using heparin and warfarin. In our four cases, direct oral anticoagulants were introduced after MT in all four patients, and Morinaga et al. [15] reported that dabigatran (Boehringer Ingelheim, Ingelheim, Germany) was introduced in two patients, and there was no recurrence for 12 months. However, the incidence of stroke after pulmonary lobectomy varies from one day to seven years, and there is currently no consensus on the appropriate duration of anticoagulation [5,16]. Three cases occurred within one week after pulmonary lobectomy, so rtPA was not available and there was a significant role for MT. Although the postoperative risk of cerebral infarction after lung cancer surgery is not widespread in the field of stroke, it is necessary to continue providing warnings of stroke.
This study has the following limitations. First, the presence of a thrombus in the PVS was not proven in three cases. Second, contrast-enhanced CT scans of the chest could not be performed in two cases after MT. Third, we have no data on cases of cerebral infarction after lung cancer surgery that were treated with conservative therapy.
We present a case series of patients who underwent MT due to cerebral embolisms after left pulmonary lobectomy. Thrombus in PVS is the probable cause of cerebral embolism, and MT is an effective treatment. Although this is a rare condition, neuroendovascular surgeons should be aware of the possibility of stroke after pulmonary lobectomy. Further studies are needed to determine its preventive and perioperative management.
Notes
REFERENCES
1. Yamamoto T, Suzuki H, Nagato K, Nakajima T, Iwata T, Yoshida S, et al. Is left upper lobectomy for lung cancer a risk factor for cerebral infarction? Surg Today. 2016; 46:780–784.

2. Kam PC, Calcroft RM. Peri-operative stroke in general surgical patients. Anaesthesia. 1997; 52:879–883.

3. Ikeda H, Yamana N, Murata Y, Saiki M. Thrombus removal by acute-phase endovascular reperfusion therapy to treat cerebral embolism caused by thrombus in the pulmonary vein stump after left upper pulmonary lobectomy: case report. NMC Case Rep J. 2014; 2:26–30.

4. Ohtaka K, Hida Y, Kaga K, Kato T, Muto J, Nakada-Kubota R, et al. Thrombosis in the pulmonary vein stump after left upper lobectomy as a possible cause of cerebral infarction. Ann Thorac Surg. 2013; 95:1924–1928.

5. Gual-Capllonch F, Teis A, Palomeras E. Pulmonary vein spontaneous echocontrast and stroke after pulmonary lobectomy. J Clin Ultrasound. 2013; 41:321–322.

6. Ohtaka K, Takahashi Y, Uemura S, Shoji Y, Hayama S, Ichimura T, et al. Blood stasis may cause thrombosis in the left superior pulmonary vein stump after left upper lobectomy. J Cardiothorac Surg. 2014; 9:159.

7. Xin Y, Hida Y, Kaga K, Iimura Y, Shiina N, Ohtaka K, et al. Left lobectomy might be a risk factor for atrial fibrillation following pulmonary lobectomy. Eur J Cardiothorac Surg. 2014; 45:247–250.

8. Nojiri T, Maeda H, Takeuchi Y, Funakoshi Y, Kimura T, Maekura R, et al. Predictive value of B-type natriuretic peptide for postoperative atrial fibrillation following pulmonary resection for lung cancer. Eur J Cardiothorac Surg. 2010; 37:787–791.

9. Ohtaka K, Hida Y, Kaga K, Takahashi Y, Kawase H, Hayama S, et al. Left upper lobectomy can be a risk factor for thrombosis in the pulmonary vein stump. J Cardiothorac Surg. 2014; 9:5.

10. Nakano T, Kaneda H, Kawaura T, Kitawaki T, Murakawa T. Ligating the pulmonary vein at the pericardial reflection is useful for preventing thrombus formation in the pulmonary vein stump after left upper lobectomy. Gen Thorac Cardiovasc Surg. 2019; 67:450–456.

11. Pillai JB, Barnard S. Cardiac tamponade: a rare complication after pulmonary lobectomy. Interact Cardiovasc Thorac Surg. 2003; 2:657–659.

12. Matsumoto K, Sato S, Okumura M, Niwa H, Hida Y, Kaga K, Committee for Patient Safety, Quality Management of Japanese Association for Chest Surgery, et al. Left upper lobectomy is a risk factor for cerebral infarction after pulmonary resection: a multicentre, retrospective, case-control study in Japan. Surg Today. 2020; 50:1383–1392.

13. Usui G, Matsumoto J, Hashimoto H, Katano T, Kusakabe M, Horiuchi H, et al. Thrombus reformation in the pulmonary vein stump confirmed 16 months after cerebral embolism on the day after left upper lobectomy for lung cancer. J Stroke Cerebrovasc Dis. 2018; 27:e225–e227.

14. Xie N, Meng X, Wu C, Lian Y, Wang C, Yu M, et al. Both left upper lobectomy and left pneumonectomy are risk factors for postoperative stroke. Sci Rep. 2019; 9:10432.

Fig. 1.
(A) Diffusion-weighted imaging showing acute ischemic stroke in the left caudate head and insular ribbon (arrows). (B) Fluid-attenuated inversion recovery showing hyperintense vessels in the middle cerebral artery (arrowheads). (C) Magnetic resonance angiography showing complete occlusion of the left internal cerebral artery (ICA) (arrow). (D) Left carotid angiography showing left ICA occlusion. (E) Left carotid angiography showing complete reperfusion. (F) Axial chest contrast-enhanced computed tomography showing a suspected thrombus in the pulmonary vein stump (arrowhead).
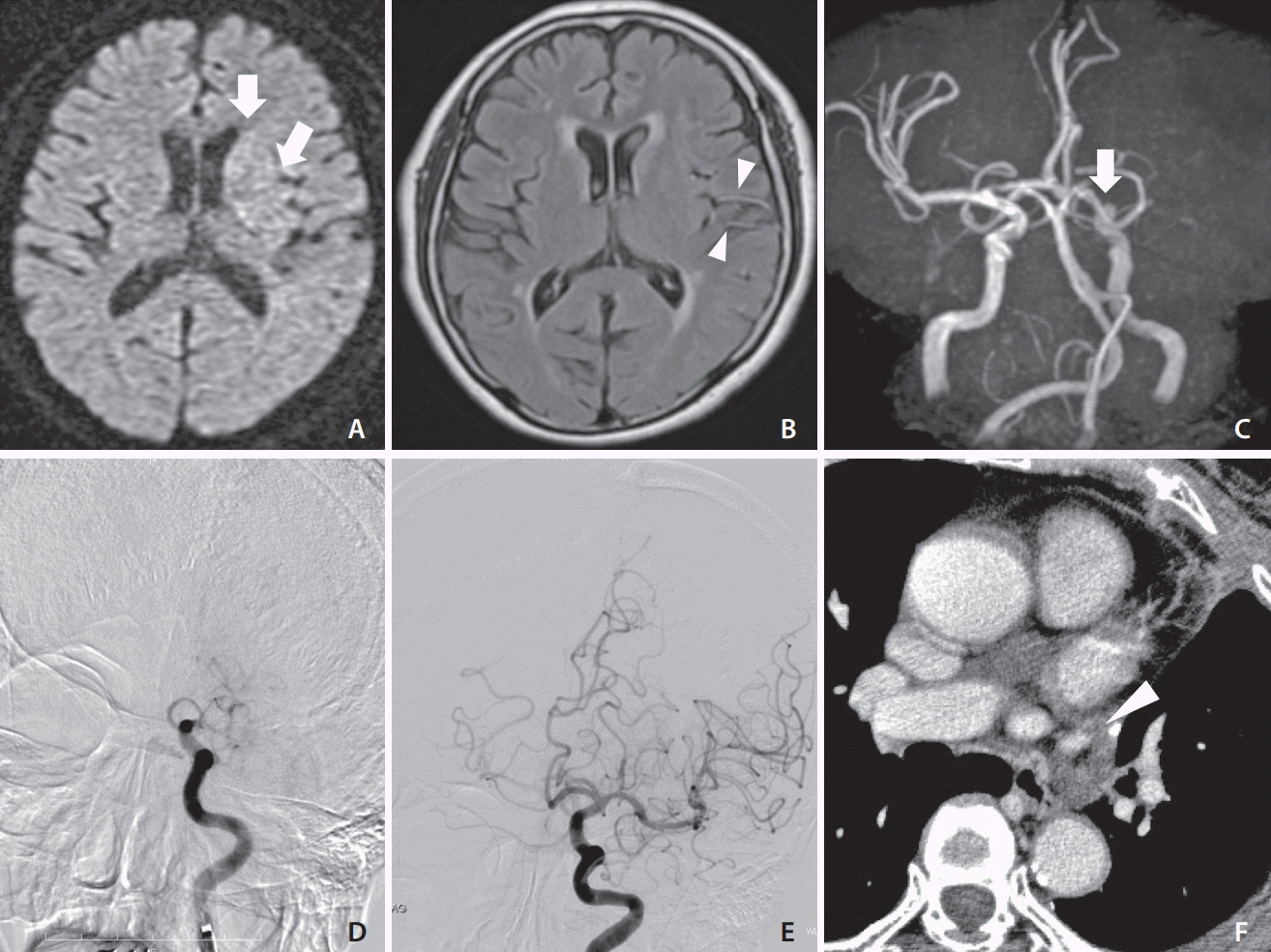
Fig. 2.
(A) Diffusion-weighted imaging (DWI) showing hyperintense signals in the right deep white matter, M4 and M5 evaluated with diffusion imaging (arrow). (B) Magnetic resonance angiography showing occlusion of the right middle cerebral artery (MCA) (arrow). (C) Right carotid angiography showing left MCA occlusion. (D) Right carotid angiography showing complete reperfusion after aspiration. (E) Coronal chest contrast-enhanced computed tomography showing no thrombus in the pulmonary vein stump. DWI-ASPECTS, Diffusion-Weighted Imaging-Alberta Stroke Program Early Computed Tomography Scores.
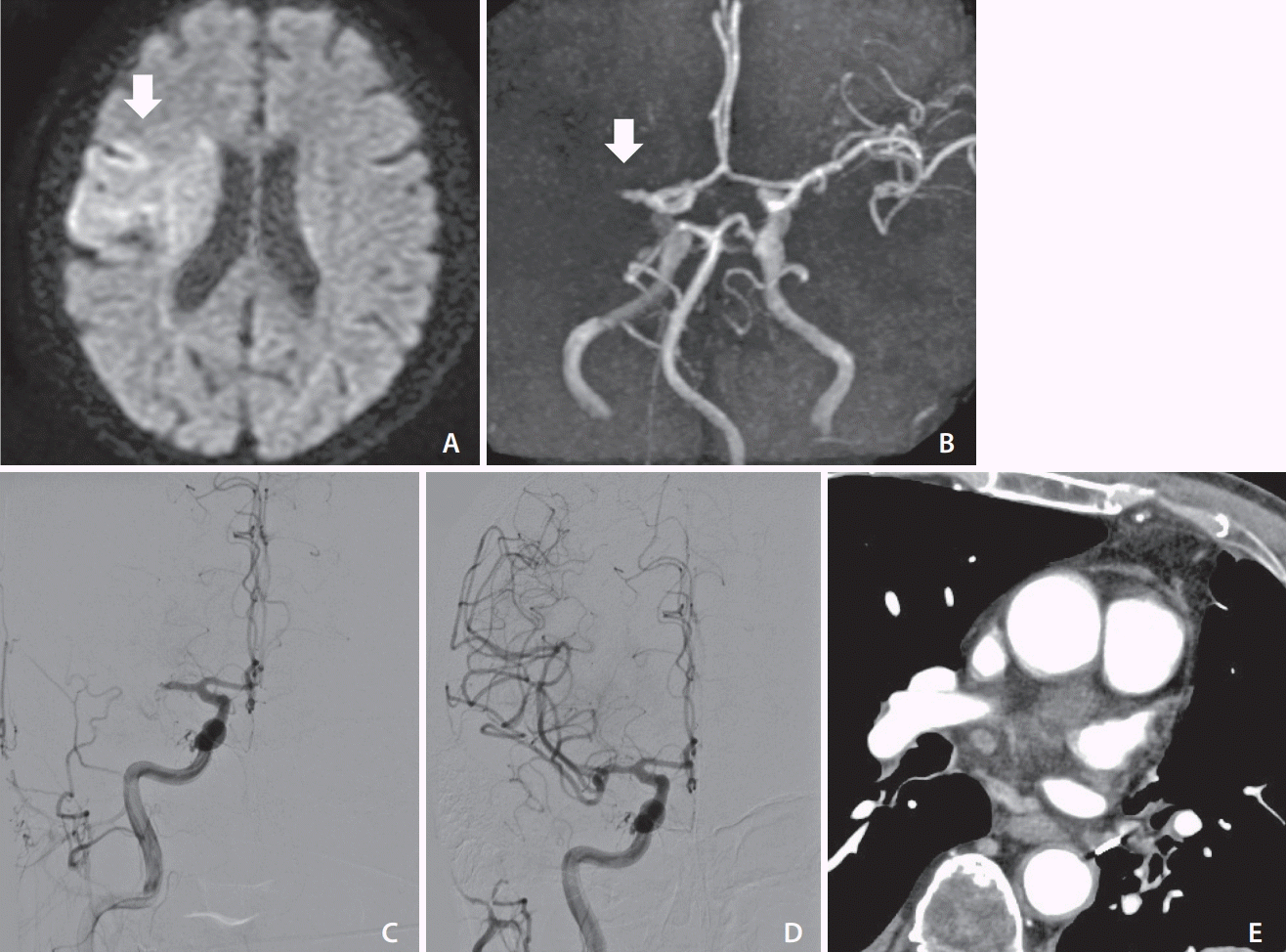
Table 1.
Summary of clinical characteristics of patients who underwent mechanical thrombectomy
NIHSS, National Institutes of Health Stroke Scale; DWI-ASPECTS, Diffusion-Weighted Imaging-Alberta Stroke Program Early Computed Tomography Scores; O2P, onset to puncture; P2R, puncture to reperfusion; TICI, thrombolysis in cerebral infarction; sICH, symptomatic intracerebral hemorrhage; mRS, modified Rankin Scale; M1, M1 segment of the middle cerebral artery; VATS, video-assisted thoracoscopic surgery; LUL, left-sided upper lobectomy; LLL, left-sided lower lobectomy; ICA, internal carotid artery.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download