1. Bettencourt S, Ferro JM. Acute ischemic stroke treatment in infective endocarditis: systematic review. J Stroke Cerebrovasc Dis. 2020; 29:104598.

2. Al-Mufti F, Schirmer CM, Starke RM, Chaudhary N, De Leacy R, Tjoumakaris SI, SNIS Standards and Guidelines Committee and SNIS Board of Directors, et al. Thrombectomy in special populations: report of the Society of NeuroInterventional Surgery Standards and Guidelines Committee. [published online ahead of print Jul 8, 2021]. J Neurointerv Surg. 2021.

3. Sonneville R, Mourvillier B, Bouadma L, Wolff M. Management of neurological complications of infective endocarditis in ICU patients. Ann Intensive Care. 2011; 1:10.

4. Kyaw H, Raju F, Shaikh AZ, Lin AN, Lin AT, Abboud J, et al. Staphylococcus lugdunensis endocarditis and cerebrovascular accident: a systemic review of risk factors and clinical outcome. Cureus. 2018; 10:e2469.

5. Elsaghir H, Al Khalili Y. Septic emboli. In : Abai B, Abu-Ghosh A, Acharya AB, Acharya U, Adhia SG, Sedeh PA, editors. StatPearls. Treasure Island (FL): StatPearls Publishing;2022.
6. Stawicki SP, Firstenberg MS, Lyaker MR, Russell SB, Evans DC, Bergese SD, et al. Septic embolism in the intensive care unit. Int J Crit Illn Inj Sci. 2013; 3:58–63.

7. Bain MD, Hussain MS, Gonugunta V, Katzan I, Gupta R. Successful recanalization of a septic embolus with a balloon mounted stent after failed mechanical thrombectomy. J Neuroimaging. 2011; 21:170–172.

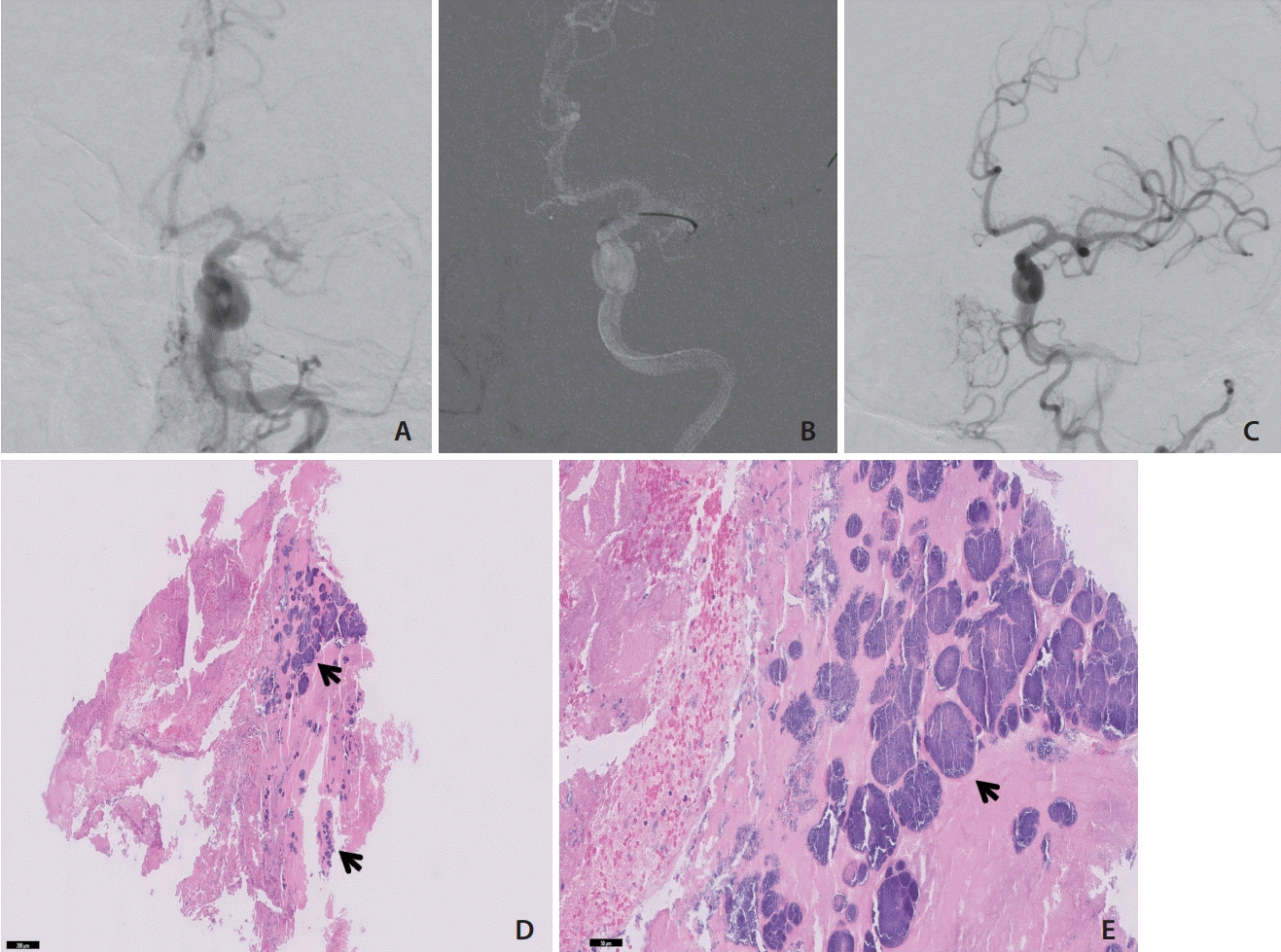
8. Hernández-Fernández F, Rojas-Bartolomé L, García-García J, Ayo-Martín Ó, Molina-Nuevo JD, Barbella-Aponte RA, et al. Histopathological and bacteriological analysis of thrombus material extracted during mechanical thrombectomy in acute stroke patients. Cardiovasc Intervent Radiol. 2017; 40:1851–1860.

9. Sabe MA, Shrestha NK, Gordon S, Menon V. Staphylococcus lugdunensis: a rare but destructive cause of coagulase-negative staphylococcus infective endocarditis. Eur Heart J Acute Cardiovasc Care. 2014; 3:275–280.

10. Ishidou M, Kanno K, Murata M, Hirose K, Ikai A, Sakamoto K. Fatal septic embolism due to Staphylococcus lugdunensis-induced bacteremia. Gen Thorac Cardiovasc Surg. 2021; 69:993–995.

11. Shah K, Jobanputra Y, Sharma P. Recurrent bacteremia in the setting of a coronary artery fistula. Cureus. 2020; 12:e9289.

12. Marnat G, Sibon I, Gory B, Richard S, Olindo S, Consoli A, ETIS Registry Investigators, et al. Safety and outcomes of mechanical thrombectomy for acute stroke related to infective endocarditis: a case-control study. Int J Stroke. 2021; 16:585–592. Erratum in: Int J Stroke 2021;16:NP3.

13. Feil K, Küpper C, Tiedt S, Dimitriadis K, Herzberg M, Dorn F, GSR Investigators, et al. Safety and efficacy of mechanical thrombectomy in infective endocarditis: a matched case-control analysis from the German stroke registry-endovascular treatment. Eur J Neurol. 2021; 28:861–867.

14. Holland TL, Baddour LM, Bayer AS, Hoen B, Miro JM, Fowler VG Jr. Infective endocarditis. Nat Rev Dis Primers. 2016; 2:16059.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download