This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
The purpose of this study was to evaluate the safety and effectiveness of a new angiographic system (Catheter 3.0 system) using a 5 French (Fr), large-bore angiography catheter, a 0.032-inch stiff guidewire, and a continuous flushing system in diagnostic cerebral angiography.
Materials and Methods
This retrospective study included 30 consecutive patients who underwent transfemoral cerebral angiography using the Catheter 3.0 system from October 2019 to March 2020. As the control group, we included 30 consecutive patients examined before the Catheter 3.0 system was introduced. Procedural outcomes, including technical success, procedure time, dose metrics, procedure-related complications, and image quality were reviewed and analyzed.
Results
All transfemoral cerebral angiographies were performed for a diagnosis of unruptured intracranial aneurysms. The Catheter 3.0 system showed a significantly shorter fluoroscopy time (6.2 vs. 9.7 minutes, P=0.008) and lower fluoroscopy dose (387.2 vs. 614.4, P=0.002) compared with the conventional 4-Fr catheter system. The Catheter 3.0 system also showed better results in terms of procedural time (21.0 vs. 22.5 minutes, P=0.072) and technical success rate (98.1% vs. 94.0%, P=0.078), although a statistical significance was not reached. The complication rate and qualitative assessment of the digital subtraction angiography (DSA) image quality were similar between the two groups.
Conclusion
The Catheter 3.0 system using a 5 Fr catheter with a large inner diameter was convenient, effective, and safe compared with the conventional system in diagnostic cerebrovascular angiography.
Keywords: Cerebral angiography, Catheters, Intracranial aneurysm
INTRODUCTION
Despite many advances in non-invasive imaging modalities such as computed tomography or magnetic resonance angiography, catheter angiography remains the gold standard for the diagnosis of cerebrovascular diseases [
1]. Conventional diagnostic catheters have an outer diameter of 4 French (Fr) or 5 Fr and have an inner diameter of 0.041 and 0.043 inches, respectively. Insufficient space between the catheter lumen and a conventional 0.035-inch guidewire leads to the requirement of guidewire removal for contrast injection, which entails several problems [
2,
3]. As an example, catheter tip malposition or instability, which may occur after the guidewire removal or during high-pressure contrast injection, can cause serious safety problems such as arterial dissection or plaque dislodgement, and lead to suboptimal image quality due to insufficient contrast volume. In addition, cleaning the catheter lumen by withdrawing blood with a syringe is necessary to remove any potential blood clots or air bubbles whenever the guidewire is removed, which prolongs the procedure time and complicates the procedure.
Our neurointervention suite developed a new angiographic system using a 5 Fr, large-bore (0.054 inches of inner diameter) angiography catheter, a 0.032-inch stiff guidewire, and a continuous flushing system with a hemostatic valve and a pressurized saline bag. We named the system as the Catheter 3.0 system, which refers to this being the 3rd generation of the angiographic catheter system after the 1st generation that used a catheter cut to the required length and the 2nd generation, which is the currently adopted method that uses a standardized catheter.
In our prior phantom study, several factors, including injection parameters and time-density curves of the conventional system and the Catheter 3.0 system were analyed, and reported the new system may show technical feasibility for cerebral catheter angiography [
3]. The main advantage of the new system is that the catheter can be stably and safely positioned with the guidewire inside during contrast injection, and there is no need for cumbersome work such as guidewire removal or catheter cleaning. The purpose of this study was to evaluate the safety and effectiveness of the Catheter 3.0 system in diagnostic cerebral angiography in comparison with the conventional catheter system.
MATERIALS AND METHODS
Patient Selection
This retrospective cohort study was approved by the Institutional Review Board of our institution (approval number: No. 2021-0873), which waived the need for informed consent. We included 30 consecutive patients who underwent transfemoral cerebral angiography for a diagnosis of unruptured intracranial aneurysms using the Catheter 3.0 system from October 2019 to March 2020. As the control group, we included 30 consecutive patients with the same inclusion criteria before the Catheter 3.0 system was introduced. The following patients were excluded: patients who were not available for the femoral approach (e.g., obesity, aortoiliac steno-occlusive lesion, aortic dissection), pediatric patients (less than 18 years of age), patients who had a high-density material (e.g., surgical prosthesis, embolic material) in the head, and patients who required angiography in less than 4 vessels (e.g., postoperative follow-up examination). Electronic medical records and radiologic images were reviewed for age, type of aortic arch, number of vessels examined, procedure-related complication, procedure time, fluoroscopy time, and radiation dose metrics.
Imaging Equipment and Protocols
All angiographic data were obtained using a biplane angiography machine (Artis Zee; Siemens, Forchheim, Germany). The detector entrance dose for digital subtraction angiography (DSA) and three-dimensional rotational angiography (3D-RA) was 1.82 and 0.24 μGy/frame, respectively. Copper filters were automatically applied in the range between 0.1 and 0.3 mm for fluoroscopy and DSA, while they were not applied for 3D-RA. The focal spot size was 0.3 mm for DSA and fluoroscopy and 0.4 mm for 3D-RA. The frame rate for fluoroscopy was set to a standard of 7.5 frames per second (f/s). The kVp, milliampere (mA), pulse width, and copper filter were automatically determined by the angiographic system in the fixed routine protocol.
Angiography Procedure
All procedures were performed by two attending neurointerventionists (5 and 3 years of experience each). The two operators were evenly assigned to both groups. Informed consent for an examination was obtained in all patients. The femoral arterial sheath size was 4 Fr or 5 Fr depending on the size of the catheter used. When the iliac artery showed marked tortuosity, a 5 Fr long sheath (length: 25 cm) was used. Access site hemostasis was achieved with manual compression followed by more than 3 hours of absolute bed rest and ipsilateral leg immobilization by applying a compressive hemostatic device (Easy-Presso; KM healthcare, Guri, Korea). We started to prepare the angiography systems according to the study group when finishing a femoral artery access.
Routine cerebral angiography, which consisted of both internal carotid artery (ICA) angiograms, both vertebral artery (VA) angiograms, and 3D-RA for the target lesion, was performed in all patients. If the contralateral V4 segment was sufficiently contrasted when angiography was performed in the dominant VA, the contralateral VA was not selected and only the ostium and extradural segments were evaluated in a contralateral subclavian arteriogram. The non-dominant VA was not routinely catheterized and was replaced with a subclavian arteriogram unless the contrast media was insufficient to fill the V4 segment or basilar artery from a contralateral VA injection [
4]. External carotid artery (ECA) angiograms were selectively performed for pre-operative evaluation of bypass surgery. For the selection of each artery, a roadmap was obtained from the proximal neck arteries and the catheter was placed in the target vessel using a guidewire [
5]. In cases of severe stenosis in the cervical arteries or tortuous anatomy, an angiogram was obtained in the proximal portions such as the common carotid or subclavian arteries.
Pre-determined injection flow rate and injection volume were used in both groups depending on the vessel size and anatomic variation: for ICA, 4–4.5 mL/s and 7–8 mL; for ECA, 2–2.5 mL/s and 4–5 mL; and for VA, 4–5 mL/s and 8–10 mL. The non-ionic contrast media used to acquire all angiographic images were Pamiray 300 (iopamidol 0.612 g/mL; Dongkook Pharmaceutical, Seoul, Korea) with a viscosity of 4.7 cp for the Catheter 3.0 system and Visipaque 270 mg/mL (iodixanol 0.550 g/mL; GE Healthcare, Chicago, IL, USA) with a viscosity of 6.3 cp for the conventional catheter system.
Angiographic Systems and Device Selection
Catheter 3.0 system
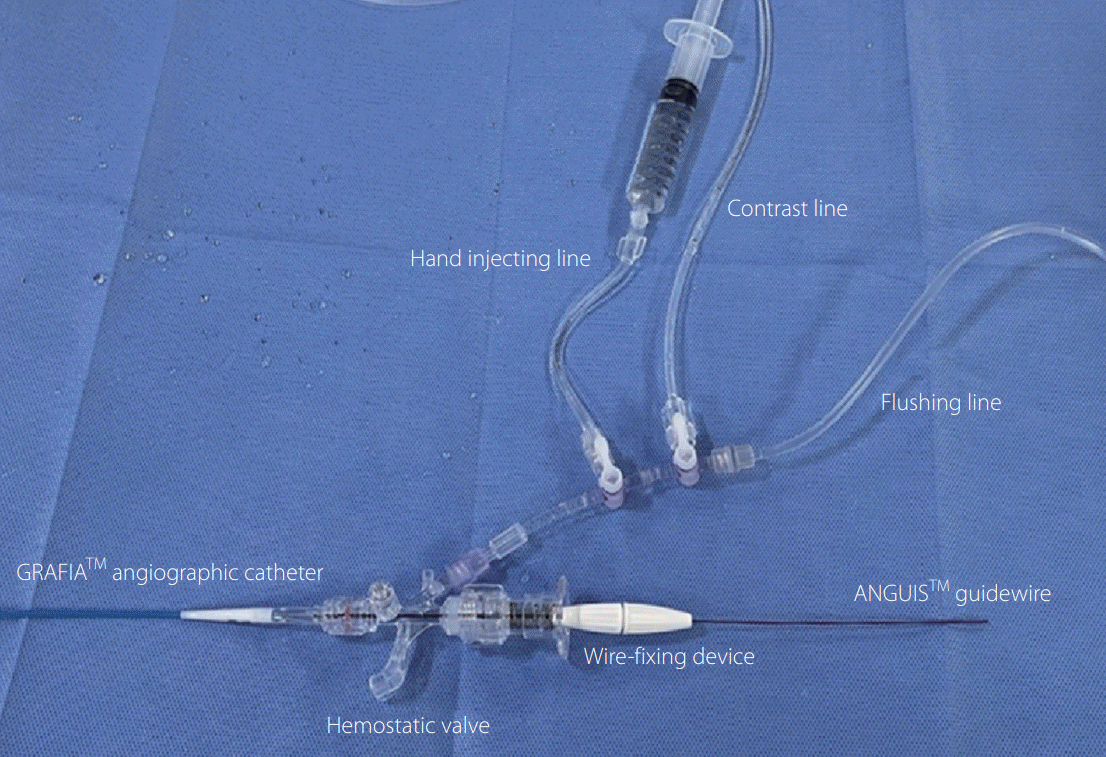
A 5-Fr angiography catheter (Grafia; Sungjin-Hitech, Suwon, Korea), a 0.032-inch guidewire (Anguis; Sungjin-Hitech), and a hemostatic valve constituted a closed system (
Fig. 1). The catheter had an inner diameter of 0.054 inches and a length of 100 cm. The catheter wall consists of three layers, including a braided stainless-steel layer to reinforce the relatively thin catheter wall compared with other angiographic catheters. The catheter consisted of several segments with varying stiffness to optimize its supportiveness and trackability. The guidewire paired with the catheter consisted of an elastic nitinol core and a black polyurethane jacket, which has an outer diameter of 0.032 inches. A hemostatic valve, which is connected to the catheter, received two lines for flushing and contrast injection, respectively, through a 3-way stopcock. The system requires an initial preparation step to remove any air in the closed system, including catheter, hemostatic valve, and contrast and flushing tubes, by aspirating a scanty amount (less than 3 mL) of blood after insertion of the catheter tip into the sheath and flushing out to the hemostatic valve. During examinzation, the system was continuously flushed using heparinized saline (2,000 U/L) with a drip infusion rate of one drop per second.
The catheter was selected at the operator’s discretion. A 5-Fr catheter with a 45-degree-angled tip was mainly used as the standard. If catheterization was not possible due to insufficient supporting of the catheter, a catheter with a stiffer and longer distal segment or a catheter with a steep angle tip was used instead. In cases of type 3 or bovine type aortic arch, a 4-Fr Simmons catheter was used as the last measure.
Conventional catheter system
The catheter system using a 4-Fr catheter (Jungsung Catheter; Jungsung Medical, Seoul, Korea) and a 0.035-inch guidewire (Glidewire; Terumo Medical Corporation, Somerset, NJ, USA) that has been routinely used in our neurovascular suite was used as a control group in this study. The catheter has an inner diameter of 0.041 inches and a length of 100 cm. The catheter was flushed intermittently by using a double-flushing technique with aspiration of a scanty amount (less than 3 mL) of blood from the catheter and clearing the catheter with 3-mL heparinized saline (5,000 U/L).
The catheter was selected at the operator’s discretion. A 4-Fr Davis or Headhunter catheter was typically used first. In cases of type 3 or bovine type aortic arch, a 4-Fr Simmons catheter was used as the last measure.
Procedural Outcomes
Effectiveness outcomes included technical success (successful catheterization of the target vessel and acquisition of more than an average of 2 points on image quality evaluation), procedure time defined as the duration between the beginning (puncture time) and the end (acquisition of the last DSA) of the procedure, and dose metrics (fluoroscopy time, fluoroscopy dose, and total radiation dose) presented in the dose report.
The safety outcome was any procedure-related complications occurring during the procedure and hospitalization period. Clinical complications included transient/permanent neurological event, contrast reaction, groin hematoma, or pseudoaneurysm. Technical complications included dissection or flow-limiting vasospasm of the carotid, vertebral, aortoiliac, and femoral arteries.
A qualitative evaluation of the image quality was performed by three fellows (1 year experience in neurointervention each). Angiographic images were randomly arranged and blinded to the reviewers. All vessels examined were evaluated using a 5-point Likert scale as follows: excellent (5-point: sufficient density ensured in all vessels, and there is a point in time when both the proximal and distal vessels were sufficiently visible at the same time); good (4-point; the density of some vessels is not sufficient, or there was no time point when all the proximal and distal vessels were sufficiently visible at the same time, but to the degree that does not significantly interfere with assessment of vascular lesions); fair (3-point; the overall density is low, and some limitations in the evaluation of small distal vessels); poor (2-point; the density is significantly lower overall, which may limit the evaluation of overall vessels), and fail (1-point; the density is significantly poor overall, hindering reliable evaluation. Request for re-examination).
Statistical Analysis
Continuous variables are expressed as mean±standard deviation or medians with interquartile ranges (IQRs). Categorical variables are expressed as frequencies with percentages. We compared the baseline characteristics and outcomes between the Catheter 3.0 and conventional catheter groups using the Student’s t-test or Wilcoxon rank-sum test (if the assumption of normality was violated on the Shapiro–Wilk normality test). Analyses of the aortic arch types and complications in each group were performed using the chi-squared test and Fisher’s exact test, respectively. The inter-rater agreement for image quality evaluation was measured by calculating the intraclass correlation coefficient (ICC) [
6]. All P-values were two-sided and values <0.05 were considered statistically significant. Statistical analyses were performed using SPSS Statistics version 28 (IBM Corp., Armonk, NY, USA).
RESULTS
The baseline characteristics of the patients are presented in
Table 1. The mean age was younger in the Catheter 3.0 group than in the conventional catheter group (62.1
vs. 67.3 years, P=0.016). The distribution of the aortic arch type was not significantly different between the two groups (P=0.29). The average number of vessels tested per patient was higher in the Catheter 3.0 group, albeit without statistical significance (5.37
vs. 5.03, P=0.14). Two operators examined the same number of patients in each group.
Safety and efficacy outcomes were demonstrated in
Table 2. The technical success rate was higher in the Catheter 3.0 group, albeit without statistical significance (98.1%
vs. 94.0%, P=0.078). Nine technically unsuccessful cases (3 in the Catheter 3.0 group, 6 in the conventional group) were due to insufficient image quality, and all were right subclavian arteriograms to evaluate the right extradural VA. In the other 3 cases, the catheter could not be placed up to the target vessel due to tortuosity in the conventional catheter group.
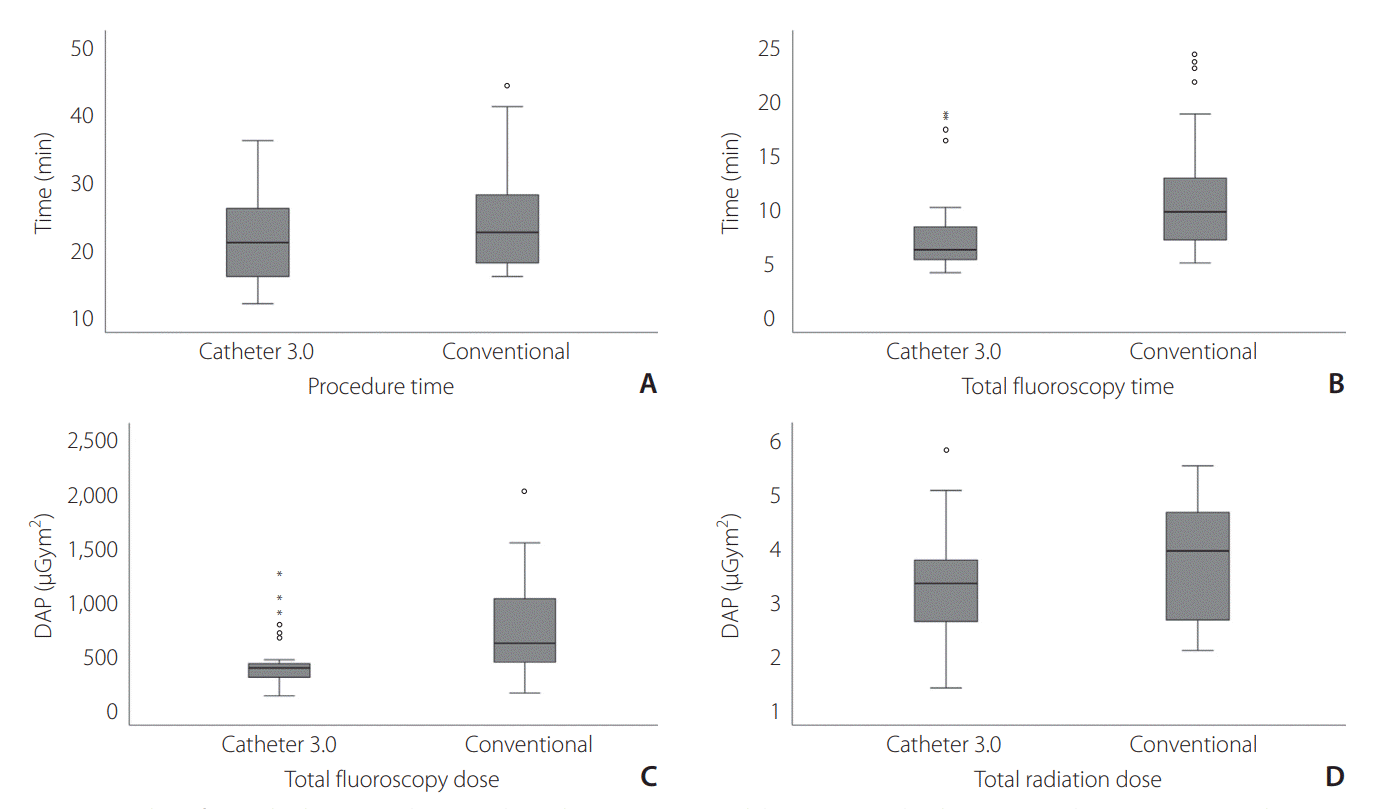
Fig. 2 shows the comparisons of procedural outcomes between the two groups. The median procedure time (
Fig. 2A) was 21.0 (IQR: 15.8–26.8) minutes in the Catheter 3.0 group, which was shorter than that in the conventional group albeit without statistical significance (22.5 [18.0–28.5] minutes; P=0.072). The median total fluoroscopy time (
Fig. 2B) was significantly shorter in the Catheter 3.0 group than in the conventional group (6.2 [5.3–8.3]
vs. 9.7 [7.1–12.8] minutes, P=0.008). Similarly, the median total fluoroscopy dose (
Fig. 2C) was significantly lower in the Catheter 3.0 group (387.2 [300.8–424.5]
vs. 614.4 [439.1–1,022.5] µGym
2, P=0.002). The mean total radiation dose (
Fig. 2D), including DSA and 3D-RA doses, was not significantly different between the two groups (P=0.15).
No clinical complications occurred in the study patients. Technical complications occurred in 6 patients overall without a significant difference between the two groups, and all were asymptomatic vasospasms. The vasospasms occurred in 2 external carotid arteries (1.2%) out of 161 vessels in the Catheter 3.0 group, and 4 (2.6%) out of 151 vessels (2 internal arteries, 2 external carotid arteries) in the conventional group.
The average number of catheters and guidewires used in the examination was lower in the Catheter 3.0 group, albeit without statistical significance. Additional catheters were used in 5 patients (16%) in the conventional group, one of whom required 3 catheters. In the Catheter 3.0 group, 4-Fr Simmons catheter was used in one patient; in contrast, a stiff guidewire was additionally used in one patient in the conventional group. In the qualitative assessment, there was no significant difference in terms of image quality between the two groups (4.08 vs. 4.02, P=0.49). The inter-rater agreement was considered to be good based on the ICC estimates (0.83; 95% confidence interval, 0.79 to 0.86).
DISCUSSION
In the current study, the Catheter 3.0 system with a large-bore 5-Fr catheter showed a significantly shorter fluoroscopy time and lower fluoroscopy dose compared with the conventional 4-Fr catheter system. The Catheter 3.0 system also showed better results in terms of procedural time and technical success rate, although a statistical significance was not reached. The complication rate and qualitative assessment of the DSA image quality were similar between the two groups.
The 5-Fr catheter of the Catheter 3.0 system resembles a guiding catheter system used in interventional procedures, which has thinner walls, larger lumens, and stiffer shafts compared with conventional angiography catheters. Therefore, the Catheter 3.0 system may have decreased catheter wall strength, increased catheter kinking, or less torque control. However, the Catheter 3.0 system achieved a higher technical success rate than the conventional system in the current study, which suggests that the mechanical property of the catheter and the guidewire was appropriately optimized and balanced between the supportiveness and the trackability, thereby resulting in an effective catheterization. In the subgroup analysis according to the aortic arch type, the Catheter 3.0 system showed better technical outcomes than the conventional system, even in cases with tortuous anatomy.
In the conventional system, the guidewire had to be removed and the catheter had to be flushed in order to safely inject the contrast media. In contrast, in the Catheter 3.0 system, the contrast media can be injected with a guidewire inside the large-bore 5-Fr catheter. In the current study, however, the Catheter 3.0 system did not show a statistically significant reduction in the procedure time as we expected. This is probably due to the insufficient number of samples to achieve statistically significant differences. In addition, the initial preparation required for the clearance of air bubbles in the Catheter 3.0 system may have offset the time-saving effect of omitting the processes of guidewire removal/re-insertion and contrast injection line connection/disconnection.
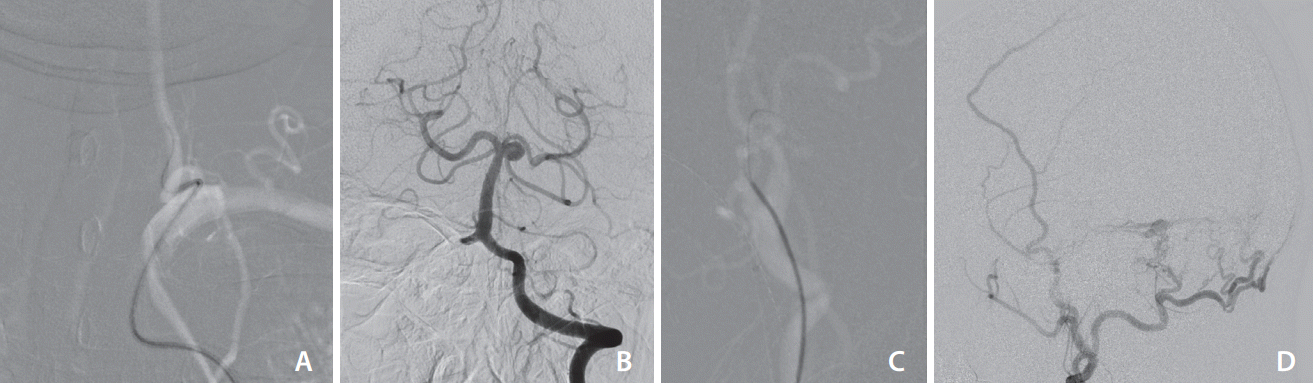
The fluoroscopy time was significantly shorter in the Catheter 3.0 group, which suggests that the processes under the fluoroscopy (navigation of the guidewire or catheter advancement) consumed less time with the Catheter 3.0 system. It can also be interpreted that the catheter could be placed in the desired location faster and probably with fewer attempts considering the smaller number of catheters and guidewires used in the Catheter 3.0 group. Additionally, the guidewire that was left in the catheter during contrast injection may have stabilized the catheter even in the unstable position and reduced the chances of excessive catheterization, catheter kickback, and contrast reflux (
Fig. 3). The fluoroscopy dose of the Catheter 3.0 system was also significantly less, which is an expected result because the fluoroscopy dose would be proportional to the fluoroscopy time if other conditions were constant.
In terms of clinical complications, we thought that fewer neurologic complications would occur in the Catheter 3.0 system group with a closed system that could reduce blood clots and air embolism. On the other hand, the possibility of puncture site problem was expected to be higher in the Catheter 3.0 system group with a larger femoral sheath size. However, there was no significant difference of clinical complications between the two groups because no clinical events were reported in both groups. Lee et al. [
7] conducted a randomized controlled trial to evaluate the influence of the two flush methods on transfemoral cerebral angiography. They also reported no procedure-related complications and neurological deterioration in both groups. These results are probably due to the overall low prevalence of clinical complications, which have been reported as 1% to 2% in previous literatures [
1,
8-
13].
Technical complications, which were all asymptomatic vasospasms, were noted in 1.2% and 2.6% of patients in the Catheter 3.0 group and the conventional group, respectively. Iatrogenic vasospasms and dissections, which are usually self-limiting and asymptomatic [
4,
14], can occur during catheterization and contrast injection and are likely associated with the movement of catheters to an undesired position or direction after guidewire removal. The occurrence of vasospasm suggests that there was a minor intimal injury; however, depending on the intensity, arterial dissection may have occurred in rare cases [
14]. In the Catheter 3.0 system, the guidewire consistently stabilizes the catheter tip in accordance with the direction of the vessel until completion of the contrast injection; therefore, the chance of intimal injury was expected to be minimized. However, since dissection is a rare complication observed with a frequency of 0.1% to 0.6% [
14], more patients are required to confirm a significant result.
The viscosity of contrast materials may be an issue in the Catheter 3.0 system. The cross-section area of the contrast-injectable space (0.96 mm2) between the catheter lumen and the 0.032-inch guidewire is larger than that (0.85 mm2) of the 4-Fr conventional catheter. However, the resistance during contrast material injection is higher in the Catheter 3.0 system because of its larger surface area, which is more than double compared with the 4-Fr catheter system. Therefore, it is recommended to use a contrast agent with as low viscosity as possible to minimize the pressure on the system during the injection with a power injector and to facilitate manual injection.
There were several limitations. First, this study had a retrospective nature and is therefore subject to selection bias. Second, the number of patients was too small to obtain statistically significant differences in procedural and safety outcomes between the two groups. A further large prospective study would be required. Third, the Catheter 3.0 group was significantly younger than the conventional group, which means that the catheterization might have been more difficult in the 4 Fr group; however, the aortic arch type was not significantly different between the two groups. Except for the aortic arch type, there is no information on other sites that may affect catheterization, which can be a limitation of the study. Forth, the iodine concentration of the contrast media used in the two groups was different, which could potentially affect image quality. Although there was no significant difference in image quality in the qualitative analysis of this study, quantitative analysis may be needed in future studies. Fifth, we did not routinely perform diffusion-weighted imaging in patients without neurologic symptoms; therfore, we could not evaluate silent embolic lesions. Lastly, there may be potential issues related to the high resistance during the pressure injection of the contrast agent, which could affect the image quality and durability of the Catheter 3.0 system. Quantitative analysis of the image quality and pressure monitoring in the Catheter 3.0 system would be necessary in a future study.
CONCLUSION
The Catheter 3.0 system using a 5 Fr catheter with a large inner diameter was convenient, effective, and safe compared with the conventional system in diagnostic cerebrovascular angiography. Well-designed, large, prospective studies are needed to prove the superiority of the Catheter 3.0 system in terms of technical success, effectiveness, and safety outcomes.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download