Abstract
Background
Methods
Results
SUPPLEMENTARY MATERIALS
Supplementary Table 1.
Supplementary Table 3.
Supplementary Table 4.
Supplementary Table 5.
Supplementary Fig. 1.
Supplementary Fig. 2.
Supplementary Fig. 3.
Notes
CONFLICTS OF INTEREST
Seung-Hyun Ko has been executive editor of the Diabetes & Metabolism Journal since 2022. She was not involved in the review process of this article. Otherwise, there was no conflict of interest.
ACKNOWLEDGMENTS
REFERENCES
Fig. 1.
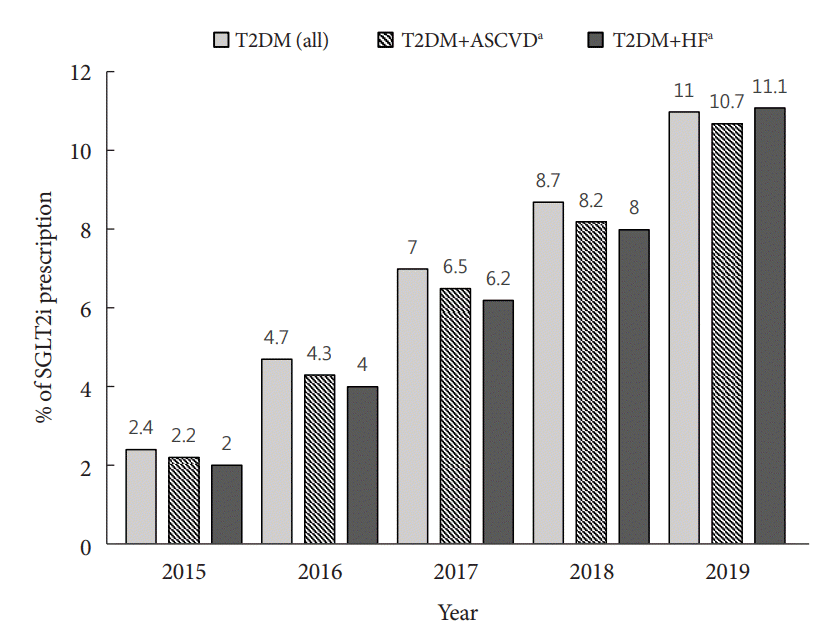
Fig. 2.
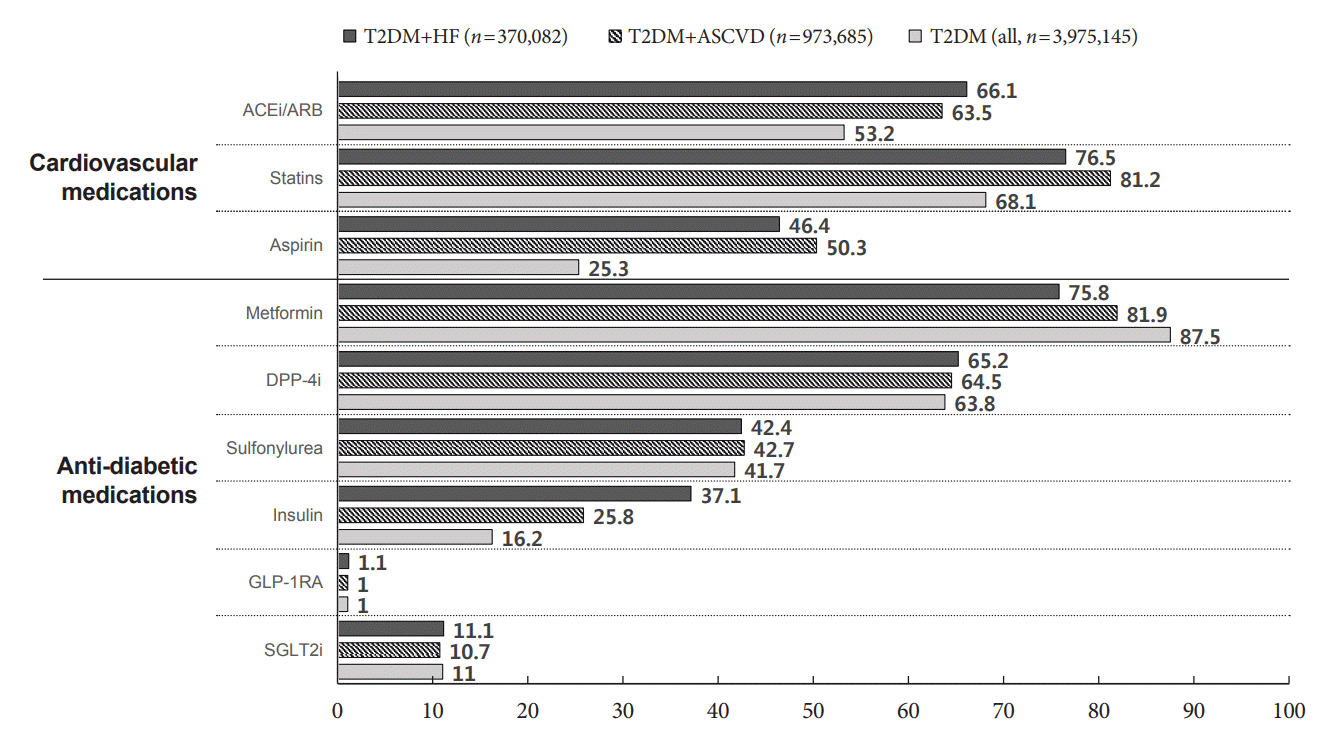
Fig. 3.
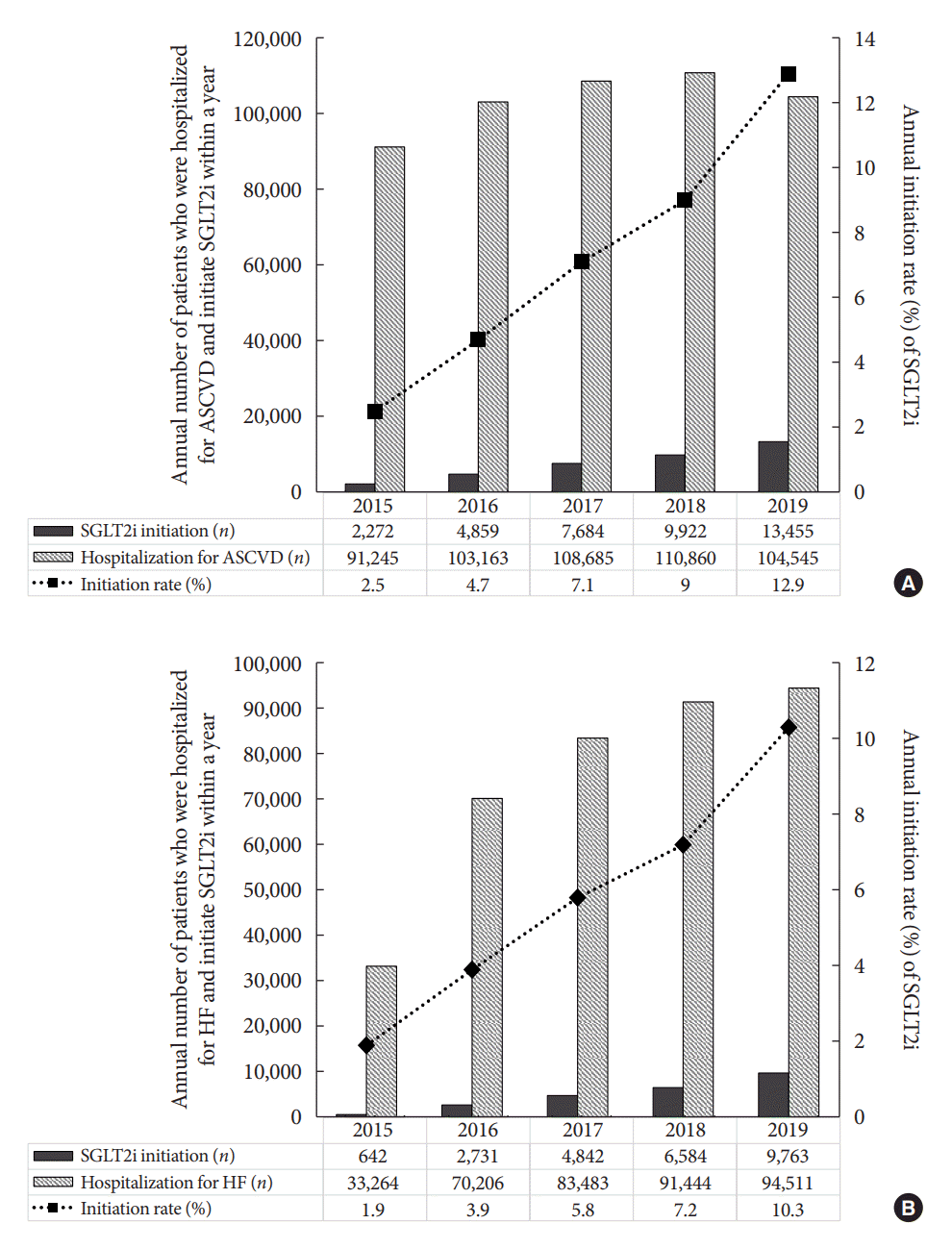
Fig. 4.
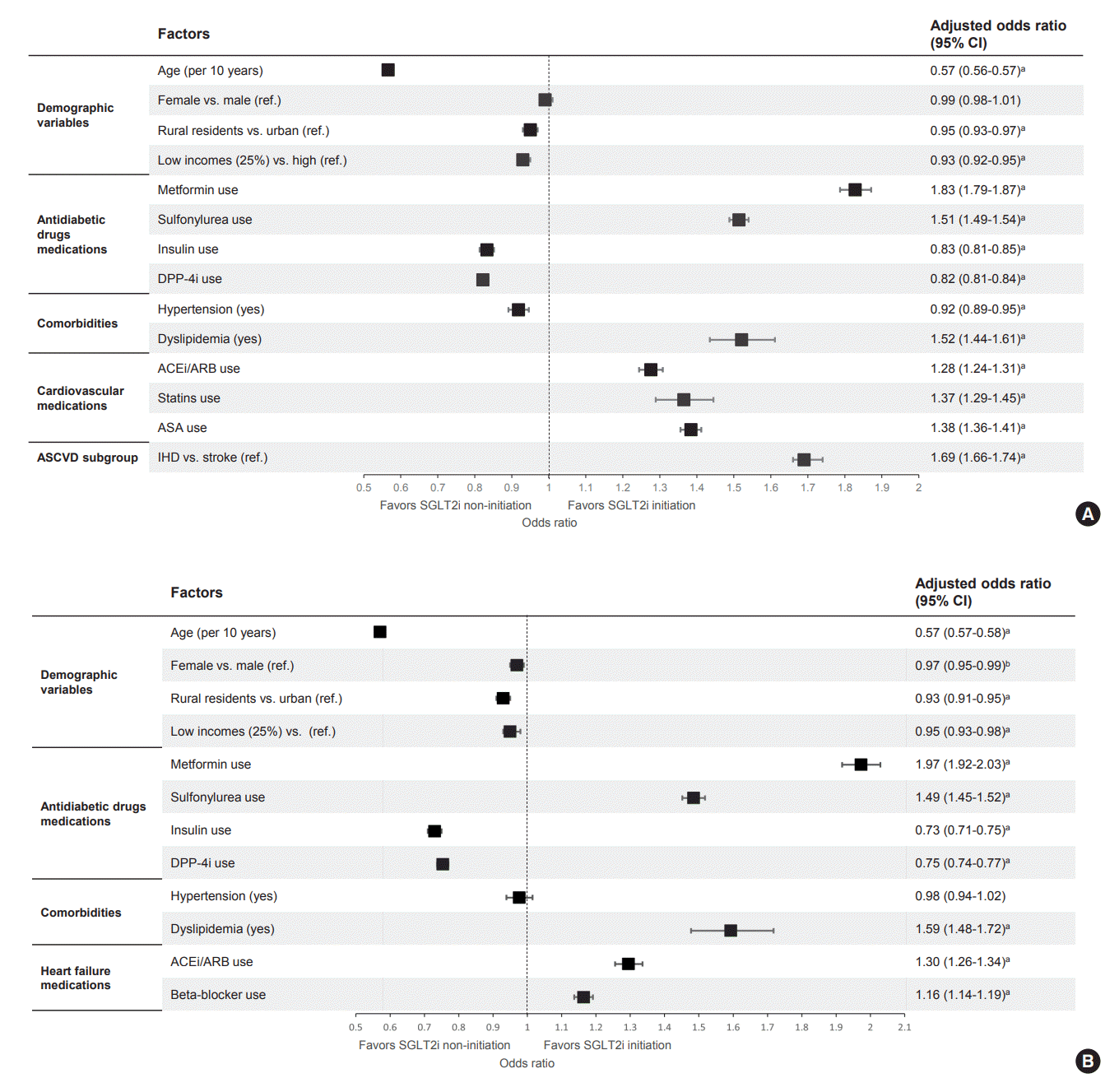
Table 1.
| Characteristic | All |
SGLT2i treatment |
P value | |
|---|---|---|---|---|
| No | Yes | |||
| Number | 518,572 | 447,313 | 71,259 | |
| Age, yr | 68.7±12.1 | 69.8±11.8 | 61.6±11.7 | <0.001 |
| Male sex | 289,347 (55.8) | 244,192 (54.6) | 45,155 (63.4) | <0.001 |
| Time to initiation, mo | - | - | 10.6 (1.2–26.8) | |
| Urban residents | 212,152 (40.9) | 182,017 (40.7) | 30,135 (42.3) | <0.001 |
| Incomes (low 25%) | 152,749 (29.5) | 132,390 (29.6) | 20,359 (28.6) | <0.001 |
| Prescription | ||||
| Hospitals | - | - | 52,332 (73.4) | |
| Clinics | - | - | 18,497 (26.0) | |
| Etc. | - | - | 430 (0.6) | |
| Comorbidities | ||||
| Hypertension | 425,037 (82.0) | 367,326 (82.1) | 57,711 (81.0) | <0.001 |
| Dyslipidemia | 393,162 (75.8) | 330,498 (73.9) | 62,664 (87.9) | <0.001 |
| Antidiabetic drugsa | ||||
| Metformin | 360,462 (69.5) | 301,817 (67.5) | 58,645 (82.3) | <0.001 |
| DPP-4i | 235,352 (45.4) | 202,327 (45.2) | 33025 (46.4) | <0.001 |
| Insulin | 134,654 (26.0) | 121,148 (27.1) | 13,506 (19.0) | <0.001 |
| Sulfonylureas | 192,135 (37.1) | 160,143 (35.8) | 31,992 (44.9) | <0.001 |
| TZD | 30,799 (5.9) | 25,912 (5.8) | 4,887 (6.9) | <0.001 |
| AGI | 14,107 (2.7) | 12,545 (2.8) | 1,562 (2.2) | <0.001 |
| Meglitinide | 3,693 (0.7) | 3,455 (0.8) | 238 (0.3) | <0.001 |
| SGLT2i | 14,516 (2.8) | 0 | 14,516 (20.4) | <0.001 |
| GLP-1RA | 833 (0.2) | 598 (0.1) | 235 (0.3) | <0.001 |
| Cardiovascular medications | ||||
| ACEi/ARB | 347,273 (67.0) | 296,864 (66.4) | 50,409 (70.7) | <0.001 |
| Statins | 393,010 (75.8) | 330,815 (74.0) | 62,195 (87.3) | <0.001 |
| ASA | 341,707 (65.9) | 289,442 (64.7) | 52,265 (73.4) | <0.001 |
Values are presented as mean±standard deviation, number (%), or median (range).
SGLT2i, sodium-glucose cotransporter 2 inhibitor; DPP-4i, dipeptidyl peptidase-4 inhibitor; TZD, thiazolidinedione; AGI, alpha-glucosidase inhibitor; GLP-1RA, glucagon-like peptide-1 receptor agonist; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockade; ASA, aspirin.
Table 2.
| Characteristic | All |
SGLT2i treatment |
P value | |
|---|---|---|---|---|
| No | Yes | |||
| Number | 372,853 | 331,136 | 41,717 | |
| Age, yr | 70.8±12.3 | 71.8±12.0 | 63.4±12.5 | <0.001 |
| Male sex | 196,458 (52.7) | 171,668 (51.84) | 24,790 (59.42) | <0.001 |
| Time to initiation, mo | - | - | 8.8 (0.3–22.5) | |
| Urban residents | 149,452 (40.1) | 132,026 (39.9) | 17,426 (41.8) | <0.001 |
| Incomes (low 25%) | 114,650 (30.8) | 101,692 (30.7) | 12,958 (31.1) | 0.142 |
| Prescription | ||||
| Hospitals | 31,419 (75.3) | |||
| Clinics | 10,048 (24.1) | |||
| Etc. | 250 (0.6) | |||
| Comorbidities | ||||
| Hypertension | 310,925 (83.4) | 275,917 (83.3) | 35,008 (83.9) | 0.002 |
| Dyslipidemia | 253,174 (67.9) | 218,124 (65.9) | 35,050 (84.0) | <0.001 |
| Antidiabetic drugsa | ||||
| Metformin | 235,753 (63.2) | 202,742 (61.2) | 33,011 (79.1) | <0.001 |
| Sulfonylureas | 130,540 (35.0) | 112,159 (33.9) | 18,381 (44.1) | <0.001 |
| DPP-4i | 171,984 (46.1) | 152,549 (46.1) | 19,435 (46.6) | 0.045 |
| Insulin | 113,093 (30.3) | 104,491 (31.6) | 8,602 (20.6) | <0.001 |
| SGLT2i | 9,841 (2.6) | 0 | 9,841 (23.6) | <0.001 |
| TZD | 23,525 (6.3) | 20,409 (6.2) | 3,116 (7.5) | <0.001 |
| AGI | 9,370 (2.5) | 8,566 (2.6) | 804 (1.9) | <0.001 |
| Meglitinide | 2,671 (0.7) | 2,554 (0.8) | 117(0.3) | <0.001 |
| HF medications | ||||
| ACEi/ARB | 247,070 (66.3) | 217,021 (65.5) | 30,049 (72.0) | <0.001 |
| Statins | 251,300 (67.4) | 216,675 (65.4) | 34,625 (83.0) | <0.001 |
| ASA | 196,245 (52.6) | 170,509 (51.5) | 25,736 (61.7) | <0.001 |
Values are presented as mean±standard deviation, number (%), or median (range).
SGLT2i, sodium-glucose cotransporter 2 inhibitor; DPP-4i, dipeptidyl peptidase-4 inhibitor; TZD, thiazolidinedione; AGI, alpha-glucosidase inhibitor; HF, heart failure; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockade; ASA, aspirin.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download