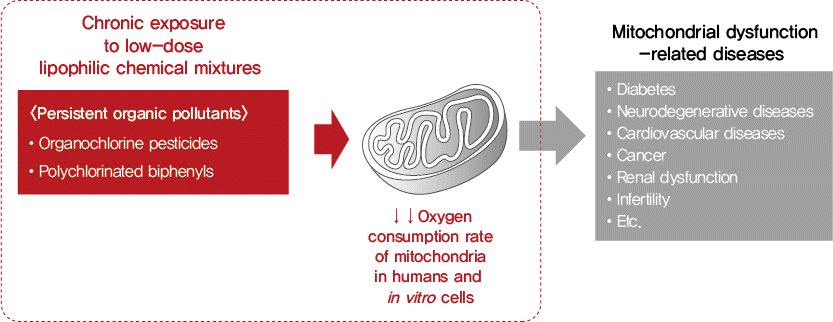
Abstract
Background
Methods
Results
SUPPLEMENTARY MATERIALS
Notes
AUTHOR CONTRIBUTIONS
Conception or design: I.K.L., D.H.L.
Acquisition, analysis, or interpretation of data: S.A.K., H.L., S.M.P., M.J.K., Y.M.L., H.K.L., H.B.M.
Drafting the work or revising: S.A.K., H.L., Y.R.Y., H.B.M., I.K.L., D.H.L.
Final approval of the manuscript: S.A.K., H.L., S.M.P., M.J.K., Y.M.L., Y.R.Y., H.K.L., H.B.M., I.K.L., D.H.L.
REFERENCES
Fig. 1
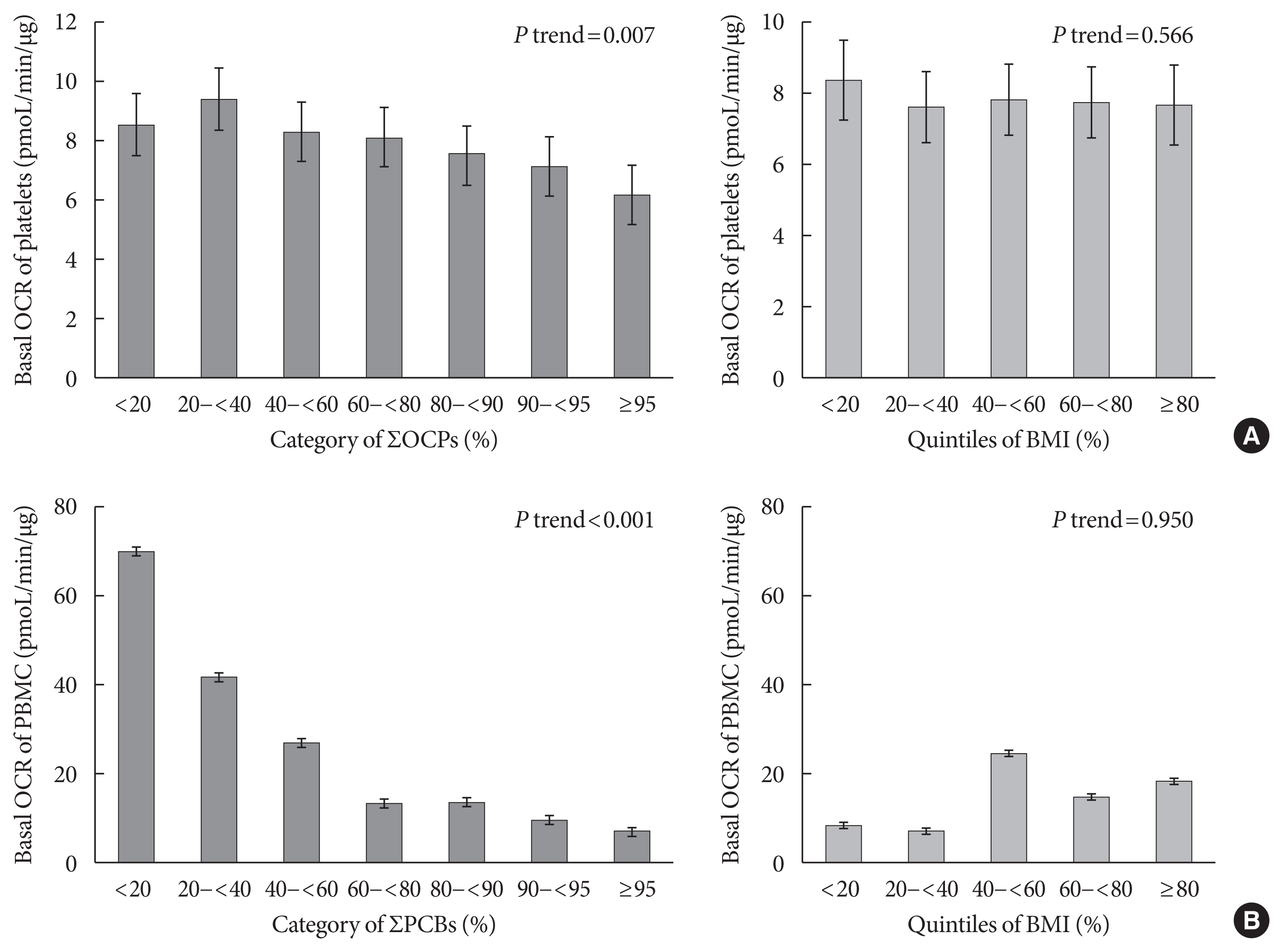
Fig. 2
Table 1
| Characteristic | Basal OCR, pmol/min/μg | |||
|---|---|---|---|---|
|
|
||||
| Platelets | P value | PBMCs | P value | |
| Age, yr | 0.593 | 0.013 | ||
| <40 | 8.9±1.1 | 19.9±1.2 | ||
| 40–49 | 8.4±1.1 | 22.3±1.2 | ||
| 50–59 | 7.0±1.1 | 19.0±1.2 | ||
| ≥60 | 8.4±1.0 | 30.6±1.1 | ||
|
|
||||
| Sex | 0.010 | 0.429 | ||
| Men | 8.9±1.0 | 25.7±1.1 | ||
| Women | 7.7±1.0 | 23.1±1.1 | ||
|
|
||||
| BMI, kg/m2a | 0.455 | 0.752 | ||
| <25 | 8.4±1.0 | 24.8±1.1 | ||
| ≥25 | 8.0±1.0 | 23.8±1.1 | ||
|
|
||||
| Current smokera | 0.554 | 0.092 | ||
| No | 8.3±1.0 | 22.6±1.1 | ||
| Yes | 8.0±1.1 | 30.4±1.2 | ||
|
|
||||
| Current drinkera | 0.474 | 0.070 | ||
| No | 8.5±1.1 | 28.8±1.1 | ||
| Yes | 8.1±1.0 | 21.9±1.1 | ||
|
|
||||
| Moderate or vigorous exercisea | 0.408 | 0.605 | ||
| No | 8.3±1.0 | 24.7±1.1 | ||
| Yes | 7.8±1.1 | 22.3±.1.2 | ||
|
|
||||
| Hypertensiona | 0.018 | 0.931 | ||
| No | 8.8±1.0 | 24.5±1.1 | ||
| Yes | 7.6±1.0 | 24.2±1.1 | ||
|
|
||||
| Type 2 diabetes mellitusa | 0.732 | 0.011 | ||
| No | 8.2±1.0 | 26.6±1.1 | ||
| Yes | 8.0±1.1 | 16.7±1.2 | ||
Table 2
Values are presented as mean±standard error. Model 1: crude; Model 2: adjusted for age and sex; Model 3: adjusted for age, sex, body mass index, hypertension, type 2 diabetes mellitus, smoking, alcohol consumption, and exercise.
OCR, oxygen consumption rate; PBMC, peripheral blood mononuclear cell; PCB, polychlorinated biphenyl; OCP, organochlorine pesticide; ∑PCBs, rank sum of five PCBs (PCB118, PCB138, PCB153, PCB180, and PCB187); ∑OCPs, rank sum of 4 OCPs (β-hexachlorocyclohexane, p,p′-dichlorodiphenyldichloroethylene, p,p′-DDT, and trans-nonachlor).
Table 3
| Variable | Basal OCR levels of platelets, pmol/min/μg | P trend | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Age, yr | ||||||
| <60 | ||||||
| Crude | 8.5±1.1 | 9.2±1.1 | 7.1±1.1 | 7.9±1.1 | 6.7±1.1 | 0.033 |
| Adjusteda | 8.1±1.1 | 9.0±1.1 | 7.3±1.1 | 8.6±1.1 | 6.9±1.1 | 0.408 |
| ≥60 | ||||||
| Crude | 9.0±1.2 | 10.1±1.1 | 9.4±1.1 | 8.0±1.1 | 7.3±1.1 | 0.011 |
| Adjusteda | 8.2±1.1 | 9.7±1.1 | 9.7±1.1 | 8.2±1.1 | 7.3±1.1 | 0.050 |
|
|
||||||
| Sex | ||||||
| Men | ||||||
| Crude | 8.6±1.1 | 10.2±1.1 | 8.8±1.1 | 10.1±1.1 | 7.0±1.1 | 0.078 |
| Adjusteda | 8.8±1.1 | 10.4±1.1 | 8.9±.1 | 10.0±1.1 | 6.8±1.1 | 0.051 |
| Women | ||||||
| Crude | 8.6±1.1 | 8.8±1.1 | 7.7±1.1 | 6.5±1.1 | 7.2±1.1 | 0.018 |
| Adjusteda | 7.7±1.1 | 8.9±1.1 | 8.1±1.1 | 6.8±1.1 | 7.3±1.1 | 0.290 |
|
|
||||||
| BMI, kg/m2 | ||||||
| <25 | ||||||
| Crude | 8.7±1.1 | 10.1±1.1 | 8.7±1.1 | 7.8±1.1 | 6.7±1.1 | 0.003 |
| Adjusteda | 8.5±1.1 | 9.8±1.1 | 8.9±1.1 | 7.9±1.1 | 6.8±1.1 | 0.028 |
| ≥25 | ||||||
| Crude | 8.5±1.1 | 8.7±1.1 | 7.7±1.1 | 8.2±1.1 | 7.7±.1.1 | 0.484 |
| Adjusteda | 8.8±1.1 | 8.8±1.1 | 7.5±1.1 | 8.2±1.1 | 7.6±1.1 | 0.405 |
|
|
||||||
| Type 2 diabetes mellitus | ||||||
| No | ||||||
| Crude | 8.7±1.1 | 9.4±1.1 | 8.1±1.1 | 7.8±1.1 | 7.0±1.1 | 0.007 |
| Adjusteda | 8.4±1.1 | 9.1±1.1 | 8.3±1.1 | 8.0±1.1 | 7.1±1.1 | 0.059 |
| Yes | ||||||
| Crude | 7.9±1.2 | 11.0±1.2 | 8.6±1.2 | 8.3±1.1 | 7.3±1.2 | 0.355 |
| Adjusteda | 7.9±1.2 | 12.3±1.2 | 8.8±1.1 | 8.3±1.1 | 6.7±1.1 | 0.127 |
|
|
||||||
| Hypertension | ||||||
| No | ||||||
| Crude | 8.9±1.1 | 10.1±1.9 | 8.3±1.1 | 7.8±1.1 | 8.1±1.1 | 0.086 |
| Adjusteda | 9.0±1.1 | 9.9±1.1 | 8.6±1.1 | 7.7±1.1 | 7.9±1.1 | 0.107 |
| Yes | ||||||
| Crude | 7.6±1.2 | 8.7±1.1 | 8.1±1.1 | 8.1±1.1 | 6.3±1.1 | 0.103 |
| Adjusteda | 7.3±1.2 | 8.9±1.1 | 8.1±1.1 | 7.9±1.1 | 6.5±1.1 | 0.150 |
Table 4
| Variable | Basal OCR levels of PBMCs, pmol/min/μg | P trend | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Age, yr | ||||||
| <60 | ||||||
| Crude | 46.7±1.2 | 24.5±1.2 | 19.2±1.3 | 9.0±1.2 | 9.5±1.2 | <0.001 |
| Adjusteda | 55.3±1.2 | 26.7±1.2 | 20.7±1.2 | 7.4±1.2 | 7.5±1.2 | <0.001 |
| ≥60 | ||||||
| Crude | 45.4±1.4 | 64.5±1.3 | 37.0±1.2 | 28.5±1.2 | 17.1±1.2 | <0.001 |
| Adjusteda | 52.6±1.4 | 74.2±1.3 | 36.3±1.2 | 24.9±1.2 | 17.6±1.2 | <0.001 |
|
|
||||||
| Sex | ||||||
| Men | ||||||
| Crude | 55.1±1.3 | 35.2±.1.3 | 40.8±1.3 | 20.1±1.2 | 14.7±1.2 | <0.001 |
| Adjusteda | 103.0±1.3 | 42.9±1.3 | 37.2±1.3 | 17.1±1.2 | 11.5±1.2 | <0.001 |
| Women | ||||||
| Crude | 41.5±1.2 | 33.8±1.2 | 23.7±1.2 | 10.2±1.3 | 11.3±1.2 | <0.001 |
| Adjusteda | 47.3±1.2 | 38.9±1.2 | 21.7±1.2 | 9.1±1.2 | 9.6±1.2 | <0.001 |
|
|
||||||
| BMI, kg/m2 | ||||||
| <25 | ||||||
| Crude | 39.7±1.2 | 42.2±1.2 | 26.3±1.2 | 16.1±1.2 | 12.7±1.2 | <0.001 |
| Adjusteda | 58.5±1.2 | 45.5±1.2 | 25.7±1.2 | 13.0±1.2 | 9.8±1.2 | <0.001 |
| ≥25 | ||||||
| Crude | 61.7±1.3 | 26.1±1.3 | 34.2±1.3 | 17.1±1.3 | 13.6±1.2 | <0.001 |
| Adjusteda | 72.1±1.3 | 35.5±1.2 | 32.6±1.2 | 14.1±1.2 | 12.0±1.2 | <0.001 |
|
|
||||||
| Type 2 diabetes mellitus | ||||||
| No | ||||||
| Crude | 46.7±1.2 | 30.9±1.2 | 26.6±1.2 | 15.6±1.2 | 14.5±1.2 | <0.001 |
| Adjusteda | 66.1±1.1 | 35.3±1.1 | 24.7±1.1 | 11.8±1.2 | 11.4±1.2 | <0.001 |
| Yes | ||||||
| Crude | 39.9±2.0 | 103.5±1.6 | 65.1±1.5 | 19.4±1.3 | 11.3±1.2 | <0.001 |
| Adjusteda | 26.2±1.9 | 112.0±1.6 | 31.0±1.6 | 24.8±1.3 | 11.7±1.2 | <0.001 |
|
|
||||||
| Hypertension | ||||||
| No | ||||||
| Crude | 45.5±1.2 | 31.8±1.2 | 21.7±1.2 | 13.3±1.2 | 12.7±1.2 | <0.001 |
| Adjusteda | 62.6±1.2 | 35.7±1.2 | 22.2±1.2 | 10.7±1.2 | 9.0±1.2 | <0.001 |
| Yes | ||||||
| Crude | 49.3±1.3 | 38.2±1.2 | 45.3±1.2 | 21.4±1.2 | 13.6±1.2 | <0.001 |
| Adjusteda | 64.1±1.3 | 44.6±1.2 | 35.7±1.2 | 18.4±1.2 | 14.3±1.2 | <0.001 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download