1. Lloyd RV, Osamura RY, Kloppel G, Rosai J. WHO classification of tumours of endocrine organs. 4th ed. Lyon: International Agency for Research on Cancer (IARC);2017. p. 65–91.
2. Mete O. Special issue on the 2022 WHO classification of endocrine and neuroendocrine tumors: a new primer for endocrine pathology practice. Endocr Pathol. 2022; 33:1–2.

3. Bai Y, Kakudo K, Jung CK. Updates in the pathologic classification of thyroid neoplasms: a review of the World Health Organization classification. Endocrinol Metab (Seoul). 2020; 35:696–715.

4. Kakudo K, Bychkov A, Bai Y, Li Y, Liu Z, Jung CK. The new 4th edition World Health Organization classification for thyroid tumors, Asian perspectives. Pathol Int. 2018; 68:641–64.

6. Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, et al. Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol. 2022; 33:27–63.

7. Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014; 159:676–90.
8. Yoo SK, Lee S, Kim SJ, Jee HG, Kim BA, Cho H, et al. Comprehensive analysis of the transcriptional and mutational landscape of follicular and papillary thyroid cancers. PLoS Genet. 2016; 12:e1006239.

9. Asa SL, Mete O. Oncocytic change in thyroid pathology. Front Endocrinol (Lausanne). 2021; 12:678119.

10. Bruford EA, Antonescu CR, Carroll AJ, Chinnaiyan A, Cree IA, Cross NC, et al. HUGO Gene Nomenclature Committee (HGNC) recommendations for the designation of gene fusions. Leukemia. 2021; 35:3040–3.

11. Rivera M, Ricarte-Filho J, Knauf J, Shaha A, Tuttle M, Fagin JA, et al. Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol. 2010; 23:1191–200.

12. Kim TH, Lee M, Kwon AY, Choe JH, Kim JH, Kim JS, et al. Molecular genotyping of the non-invasive encapsulated follicular variant of papillary thyroid carcinoma. Histopathology. 2018; 72:648–61.

13. Lee SR, Jung CK, Kim TE, Bae JS, Jung SL, Choi YJ, et al. Molecular genotyping of follicular variant of papillary thyroid carcinoma correlates with diagnostic category of fineneedle aspiration cytology: values of RAS mutation testing. Thyroid. 2013; 23:1416–22.

14. Jung CK, Kim Y, Jeon S, Jo K, Lee S, Bae JS. Clinical utility of EZH1 mutations in the diagnosis of follicular-patterned thyroid tumors. Hum Pathol. 2018; 81:9–17.

15. Jung CK, Bychkov A, Song DE, Kim JH, Zhu Y, Liu Z, et al. Molecular correlates and nuclear features of encapsulated follicular-patterned thyroid neoplasms. Endocrinol Metab (Seoul). 2021; 36:123–33.

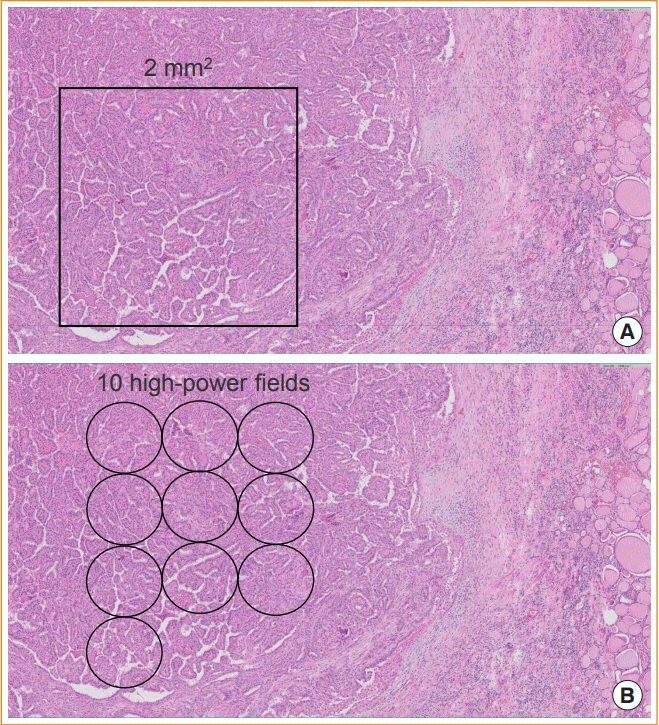
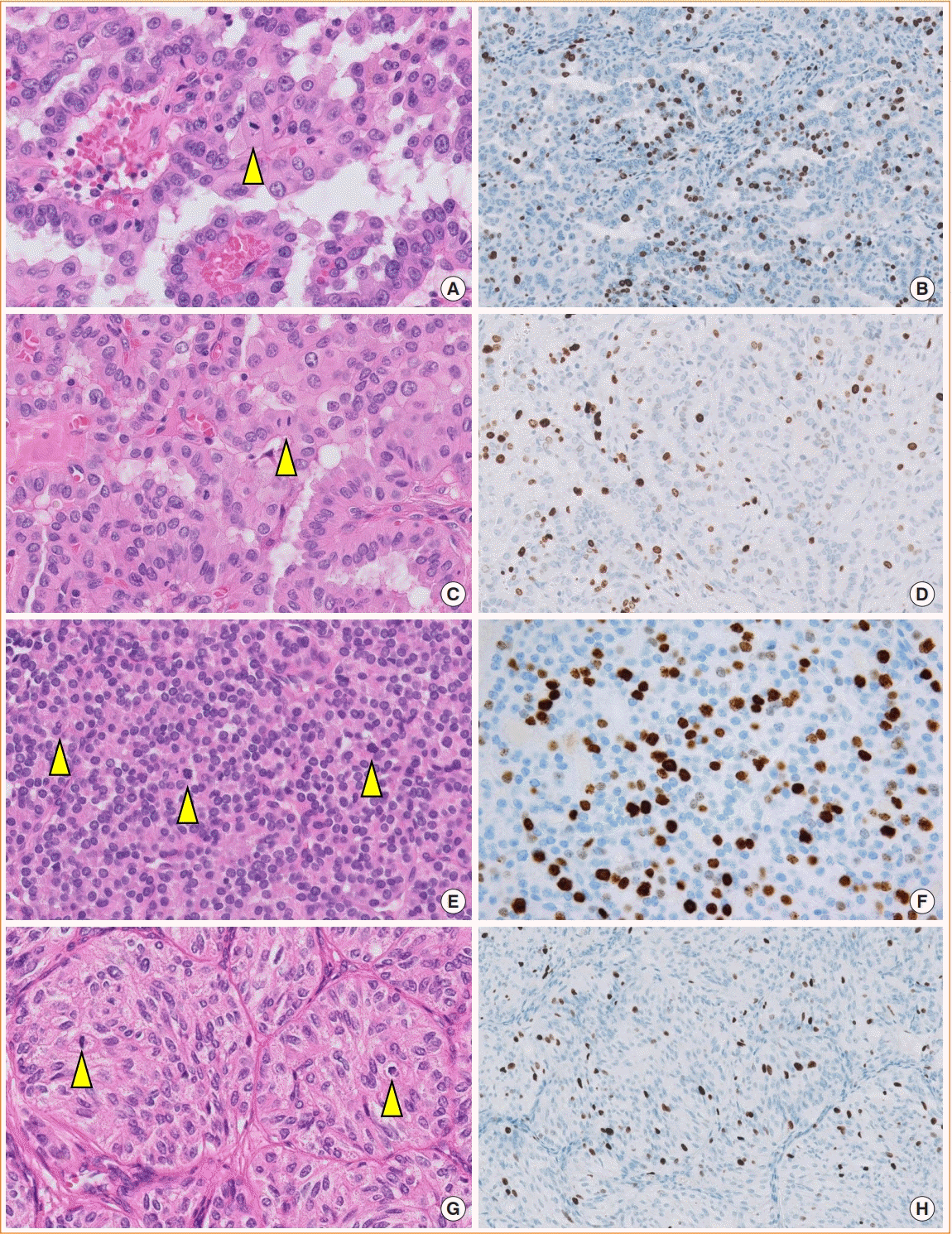
16. Cree IA, Tan PH, Travis WD, Wesseling P, Yagi Y, White VA, et al. Counting mitoses: SI(ze) matters! Mod Pathol. 2021; 34:1651–7.

17. Hellgren LS, Stenman A, Paulsson JO, Hoog A, Larsson C, Zedenius J, et al. Prognostic utility of the Ki-67 labeling index in follicular thyroid tumors: a 20-year experience from a tertiary thyroid center. Endocr Pathol. 2022; 33:231–42.

18. Cree IA. From counting mitoses to Ki67 assessment: technical pitfalls in the new WHO classification of endocrine and neuroendocrine tumors. Endocr Pathol. 2022; 33:3–5.

19. Xu B, Fuchs TL, Ahmadi S, Alghamdi M, Alzumaili B, Bani MA, et al. International medullary thyroid carcinoma grading system: a validated grading system for medullary thyroid carcinoma. J Clin Oncol. 2022; 40:96–104.

20. Calebiro D, Grassi ES, Eszlinger M, Ronchi CL, Godbole A, Bathon K, et al. Recurrent EZH1 mutations are a second hit in autonomous thyroid adenomas. J Clin Invest. 2016; 126:3383–8.

21. Cho U, Mete O, Kim MH, Bae JS, Jung CK. Molecular correlates and rate of lymph node metastasis of non-invasive follicular thyroid neoplasm with papillary-like nuclear features and invasive follicular variant papillary thyroid carcinoma: the impact of rigid criteria to distinguish non-invasive follicular thyroid neoplasm with papillary-like nuclear features. Mod Pathol. 2017; 30:810–25.

22. Nikiforov YE, Baloch ZW, Hodak SP, Giordano TJ, Lloyd RV, Seethala RR, et al. Change in diagnostic criteria for noninvasive follicular thyroid neoplasm with papillarylike nuclear features. JAMA Oncol. 2018; 4:1125–6.

23. Xu B, Serrette R, Tuttle RM, Alzumaili B, Ganly I, Katabi N, et al. How many papillae in conventional papillary carcinoma?: a clinical evidence-based pathology study of 235 unifocal encapsulated papillary thyroid carcinomas, with emphasis on the diagnosis of noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Thyroid. 2019; 29:1792–803.

24. Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016; 2:1023–9.

25. Xu B, Reznik E, Tuttle RM, Knauf J, Fagin JA, Katabi N, et al. Outcome and molecular characteristics of non-invasive encapsulated follicular variant of papillary thyroid carcinoma with oncocytic features. Endocrine. 2019; 64:97–108.

26. Xu B, Farhat N, Barletta JA, Hung YP, Biase D, Casadei GP, et al. Should subcentimeter non-invasive encapsulated, follicular variant of papillary thyroid carcinoma be included in the noninvasive follicular thyroid neoplasm with papillary-like nuclear features category? Endocrine. 2018; 59:143–50.

27. Ito Y, Hirokawa M, Miyauchi A, Higashiyama T, Kihara M, Miya A. Prognostic significance of the proportion of tall cell components in papillary thyroid carcinoma. World J Surg. 2017; 41:742–7.

28. Wong KS, Chen TY, Higgins SE, Howitt BE, Lorch JH, Alexander EK, et al. A potential diagnostic pitfall for hobnail variant of papillary thyroid carcinoma. Histopathology. 2020; 76:707–13.

29. Cameselle-Teijeiro JM, Eloy C, Sobrinho-Simoes M. Pitfalls in challenging thyroid tumors: emphasis on differential diagnosis and ancillary biomarkers. Endocr Pathol. 2020; 31:197–217.

30. Cho J, Shin JH, Hahn SY, Oh YL. Columnar cell variant of papillary thyroid carcinoma: ultrasonographic and clinical differentiation between the indolent and aggressive types. Korean J Radiol. 2018; 19:1000–5.

31. Chen JH, Faquin WC, Lloyd RV, Nose V. Clinicopathological and molecular characterization of nine cases of columnar cell variant of papillary thyroid carcinoma. Mod Pathol. 2011; 24:739–49.

32. Lam AK. Squamous cell carcinoma of thyroid: a unique type of cancer in World Health Organization Classification. Endocr Relat Cancer. 2020; 27:R177–92.

33. Lai WA, Hang JF, Liu CY, Bai Y, Liu Z, Gu H, et al. PAX8 expression in anaplastic thyroid carcinoma is less than those reported in early studies: a multi-institutional study of 182 cases using the monoclonal antibody MRQ-50. Virchows Arch. 2020; 476:431–7.

34. Chambers MA, Sadow PM, Kerr DA. Squamous differentiation in the thyroid: metaplasia, neoplasia, or bystander? Int J Surg Pathol. 2022; 30:385–92.

35. Ito Y, Miyauchi A, Nakamura Y, Miya A, Kobayashi K, Kakudo K. Clinicopathologic significance of intrathyroidal epithelial thymoma/carcinoma showing thymus-like differentiation: a collaborative study with Member Institutes of The Japanese Society of Thyroid Surgery. Am J Clin Pathol. 2007; 127:230–6.

36. Saliba M, Mohanty AS, Ho AL, Drilon A, Dogan S. Secretory carcinoma of the thyroid in a 49-year-old man treated with larotrectinib: protracted clinical course of disease despite the high-grade histologic features. Head Neck Pathol. 2022; 16:612–20.

37. Tirado Y, Williams MD, Hanna EY, Kaye FJ, Batsakis JG, El-Naggar AK. CRTC1/MAML2 fusion transcript in high grade mucoepidermoid carcinomas of salivary and thyroid glands and Warthin’s tumors: implications for histogenesis and biologic behavior. Genes Chromosomes Cancer. 2007; 46:708–15.

38. Shah AA, La Fortune K, Miller C, Mills SE, Baloch Z, LiVolsi V, et al. Thyroid sclerosing mucoepidermoid carcinoma with eosinophilia: a clinicopathologic and molecular analysis of a distinct entity. Mod Pathol. 2017; 30:329–39.

39. Agaimy A, Togel L, Stoehr R, Meidenbauer N, Semrau S, Hartmann A, et al. NSD3-NUTM1-rearranged carcinoma of the median neck/thyroid bed developing after recent thyroidectomy for sclerosing mucoepidermoid carcinoma with eosinophilia: report of an extraordinary case. Virchows Arch. 2021; 479:1095–9.

40. Wiles AB, Kraft AO, Mueller SM, Powers CN. Sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid: case report of a rare lesion with novel genetic mutation. Diagn Cytopathol. 2019; 47:589–93.

41. Hunt JL, LiVolsi VA, Barnes EL. p63 expression in sclerosing mucoepidermoid carcinomas with eosinophilia arising in the thyroid. Mod Pathol. 2004; 17:526–9.

42. Werner RG, Langlouis-Gau H, Walz F, Allgaier H, Hoffmann H. Validation of biotechnological production processes. Arzneimittelforschung. 1988; 38:855–62.
43. Noor M, Russell DK, Israel AK, Lott Limbach A. Thyroid sclerosing mucoepidermoid carcinoma with eosinophilia in conjunction with parotid basal cell adenoma: cytologic, histologic, and molecular features. Diagn Cytopathol. 2021; 49:E262–8.

44. Cameselle-Teijeiro JM, Peteiro-Gonzalez D, Caneiro-Gomez J, Sanchez-Ares M, Abdulkader I, Eloy C, et al. Cribriform-morular variant of thyroid carcinoma: a neoplasm with distinctive phenotype associated with the activation of the WNT/β-catenin pathway. Mod Pathol. 2018; 31:1168–79.

45. Boyraz B, Sadow PM, Asa SL, Dias-Santagata D, Nose V, Mete O. Cribriform-morular thyroid carcinoma is a distinct thyroid malignancy of uncertain cytogenesis. Endocr Pathol. 2021; 32:327–35.

46. Nose V. Familial thyroid cancer: a review. Mod Pathol. 2011; 24 Suppl 2:S19–33.

47. Guilmette J, Nose V. Hereditary and familial thyroid tumours. Histopathology. 2018; 72:70–81.

48. Rooper LM, Bynum JP, Miller KP, Lin MT, Gagan J, Thompson LD, et al. Recurrent DICER1 hotspot mutations in malignant thyroid gland teratomas: molecular characterization and proposal for a separate classification. Am J Surg Pathol. 2020; 44:826–33.
49. Agaimy A, Witkowski L, Stoehr R, Cuenca JC, GonzalezMuller CA, Brutting A, et al. Malignant teratoid tumor of the thyroid gland: an aggressive primitive multiphenotypic malignancy showing organotypical elements and frequent DICER1 alterations-is the term “thyroblastoma” more appropriate? Virchows Arch. 2020; 477:787–98.

50. Yang J, Sarita-Reyes C, Kindelberger D, Zhao Q. A rare malignant thyroid carcinosarcoma with aggressive behavior and DICER1 gene mutation: a case report with literature review. Thyroid Res. 2018; 11:11.

51. Wong KS, Dong F, Telatar M, Lorch JH, Alexander EK, Marqusee E, et al. Papillary thyroid carcinoma with highgrade features versus poorly differentiated thyroid carcinoma: an analysis of clinicopathologic and molecular features and outcome. Thyroid. 2021; 31:933–40.

52. Xu B, David J, Dogan S, Landa I, Katabi N, Saliba M, et al. Primary high-grade non-anaplastic thyroid carcinoma: a retrospective study of 364 cases. Histopathology. 2022; 80:322–37.

53. Rivera M, Ghossein RA, Schoder H, Gomez D, Larson SM, Tuttle RM. Histopathologic characterization of radioactive iodine-refractory fluorodeoxyglucose-positron emission tomography-positive thyroid carcinoma. Cancer. 2008; 113:48–56.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download