Abstract
Background
In this study, we evaluated the recent changes in the standardized, age-specific, stage-specific incidence rates (IRs) of thyroid cancer in Korea and compared them with the incidence data reported by the Surveillance, Epidemiology, and End Results Program.
Methods
The analysis was conducted using the incidence data (2005 to 2018) from the Statistics Korea and Korea Central Cancer Registry.
Results
The age-standardized IR (SIR) of thyroid cancer increased from 24.09 per 100,000 in 2005 to 74.83 in 2012 (annual percent change [APC], 14.5). From 2012 to 2015, the SIR decreased to 42.52 (APC, –17.9) and then remained stable until 2018 (APC, 2.1). This trend was similar in both men and women. Regarding age-specific IRs, the IRs for ages of 30 years and older showed a trend similar to that of the SIR; however, for ages below 30 years, no significant reduction was observed from the vertex of IR in 2015. Regarding stage-specific IRs, the increase was more prominent in those with regional disease (APC, 17.4) than in those with localized disease until 2012; then, the IR decreased until 2015 (APC, –16.1). The average APC from 2005 to 2018 increased in men, those under the age of 30 years, and those with regional disease.
Conclusion
The SIR in Korea peaked in 2012 and decreased until 2015 and then remained stable until 2018. However, in young individuals under the age of 30 years, the IR did not significantly decrease but tended to increase again. In terms of stage-specific IRs, the sharpest increase was seen among those with regional disease.
In recent decades, the incidence rate (IR) of thyroid cancer has risen markedly worldwide with substantial variability between geographic regions or populations [1-3]. Although this increase in incidence has predominantly been affected by the increased detection of early tumors, other risk factors, such as obesity, must have had an effect as well [2,4,5]. A growing awareness of the impacts of overdiagnosis has led to substantial modifications of national and international guidelines for the management of thyroid nodules and cancers, which now explicitly recommend against screening for thyroid cancer in asymptomatic individuals and advocate active surveillance for microcarcinomas [1,6,7].
Notably, Korea has the highest IR worldwide, reaching 45 per 100,000 according to the Global Cancer Observatory (GLOBOCAN) estimates in 2020 [8]. The number of women in Korea is 15 times greater than that in different regions of the world [8]. In Korea, the incidence of thyroid cancer had been increasing by 22.3% per year in both sexes and had been the most common cancer from 2009 to 2014 [9]. By gradually recognizing the effects of overdiagnosis, a study reported a marked decrease in thyroid operations in Korea [10]. The epidemiology and natural history of cancer can vary across populations (e.g., by geography) or among subgroups within a population [11]. This might be because of multiple causes, such as differences in diagnostic practices, healthcare systems, environmental exposures, and individual risk factors [2]. The acquisition of population-based epidemiological data on cancer burden is a fundamental attribute of a well-functioning healthcare system and will help guide future clinical recommendations and the search for contributing factors for the disease [12,13].
In this study, we evaluated recent trends in the IR of thyroid cancer by age-standardization or according to age and stage until 2018 and estimated the annual percent change (APC) in Korea. Additionally, we compared Korean incidence trends of thyroid cancer with the incidence data from the Surveillance, Epidemiology, and End Results Program (SEER).
The cancer-specific incidence data from 2005 to 2018 were obtained from Statistics Korea. The disease was coded and classified according to the code for thyroid cancer (C73) in the tenth revision of the International Statistical Classification of Diseases and Related Health Problems. Stage-specific incidence data were obtained from the Korea Central Cancer Registry. Stagespecific incidence data was available since 2005. The stages were described as follows: localized (single or multifocal invasive tumor[s] confined to the thyroid); regional (tumor[s] extending beyond the thyroid gland); distant (tumor[s] extending beyond the thyroid gland to other organs or also known as primary tumor is metastatic); and unstaged (unknown stage). Data were downloaded on July 27, 2021. We assessed SEER*explorer and downloaded the incidence data on August 2021 [14]. Informed consent was waived and the study protocol was approved by the Institutional Review Board (IRB) of Hallym University Dongtan Sacred Heart Hospital, Korea (IRB no. HDT 2022-04-015).
The crude and standardized IRs (SIRs) were calculated for thyroid cancer. The 95% confidence interval (CI) of the IR was calculated by assuming a Poisson distribution. The age-SIR was determined using the Korean mid-year resident registration population of 2,000 (defined as the standard Korean population). We sub-analyzed the IRs according to sex, age, and stage groups.
We performed joinpoint regression analysis allowing for a maximum of two joinpoints using a log-linear model to identify significant changes in IR trends [15]. For each period of the identified trends, we calculated the estimated APC. P values of less than 0.05 were used to indicate statistical significance. Statistical analyses were performed using the Joinpoint Regression Program version 4.8.0.1 (Statistical Research and Application Branch, National Cancer Institute, Bethesda, MD, USA).
The SIR of thyroid cancer was 24.09 (95% CI, 23.65 to 24.54) per 100,000 in 2005, which sharply increased to 74.83 (95% CI, 74.06 to 75.62) per 100,000 in 2012 (Table 1, Fig. 1A). After 2012, the SIR started to decrease and reached 42.52 (95% CI, 41.93 to 43.11) per 100,000 in 2015 and remained at a similar level until 2018. The APC of the SIR between 2005 and 2012 was 14.5 (95% CI, 10.5 to 18.7; P<0.01), and that between 2012 and 2015 was −17.9 (95% CI, −30.2 to −3.4; P<0.01) (Table 2, Fig. 1B). The APC of the SIR between 2015 and 2018 was 2.1 (95% CI, −9.1 to 14.6; P=0.70) and did not show statistical significance. The average APC (AAPC) from 2005 to 2018 was 3.3 (95% CI, −0.8 to 7.5; P=0.10). This SIR trend was similar in both sexes (Table 1, and Fig. 1A, C, D). In contrast, the SIRs in the SEER were not as pronounced as those in the Korean data. The SIR of thyroid cancer was 11.12 (95% CI, 10.92 to 11.32) per 100,000 in 2005, which gradually increased to 16.23 (95% CI, 16.00 to 16.46) per 100,000 in 2015. Furthermore, the trend decreased after that (Fig. 1E). The APC of the SIR between 2009 and 2014 was 2.35 (95% CI, 1.11 to 3.59) and that between 2014 and 2018 was −2.76 (95% CI, −3.91 to −1.59).
Regarding age-specific IRs, the IRs for patients aged under 20 years and those in their 20s showed a significant increase until 2013 (Table 1, Fig. 2A-C). After that, the IR tended to decrease; however, this decrease was insignificant. In patients in their 30s, the IR increased from 28.68 (95% CI, 27.58 to 29.82) in 2005 to 105.38 (95% CI, 103.16 to 107.64) in 2012 (APC, 16.7; 95% CI, 12.0 to 21.6; P<0.05) and then decreased to 65.86 (95% CI, 63.88 to 67.52) until 2015 (APC, −15.8; 95% CI, −28.1 to −1.4; P<0.05) (Tables 1, 2, Fig. 2D). In patients aged under 20 years, those in their 20s, and those in their 30s, the AAPC during the 2005 to 2018 period significantly increased (Table 2). The IR trend in patients aged 40 years and above was similar to that of the SIR (Fig. 2E-H). Furthermore, in the SEER data, in patients under the age of 15 years, the IR trend persistently increased from 2000 to 2018 (4.61; 95% CI, 3.50 to 5.80). In other age groups, the IR continued to increase until 2014 or 2015 and then declined (Fig. 2I).
Regarding stage-specific IRs, the most prominent increase was observed in patients with regional disease (Table 1, Fig. 3A). The stage-specific IR of regional disease was 9.33 (95% CI, 9.05 to 9.61) in 2005, increased to 37.95 (95% CI, 37.40 to 38.51) in 2012, and then decreased to 24.76 (95% CI, 24.31 to 25.21). The APC of the IR of regional disease was 17.4 (95% CI, 12.6 to 22.4; P<0.05) between 2005 and 2012 and −16.1 (95% CI, −27.9 to −2.4; P<0.05). The AAPC of the IR of regional disease during the 2005 to 2018 period was 4.8 (95% CI, 0.8 to 9.0; P<0.05) (Table 2, Fig. 3B). The localized and distant groups showed a trend similar to that of the SIR (Table 2, Fig. 3C, D). In contrast, SEER data have a high proportion of patients with localized disease throughout the years, and the IR seemed to have a similar increasing trend (Fig. 3E).
In this study, we investigated the recent trends of the SIR of thyroid cancer in Korea. The SIR peaked in 2012 and began to decline. This trend was the same for both sexes and for all age groups. However, since 2015, the decline in the SIR has slowed down or even tended to increase again. Of note, the IR of thyroid cancer in patients aged under 20 years and those in their 20s did not show a clear decline even after 2012, even if the absolute number of occurrences among those was small. According to the stage groups, the IR in patients with regional disease characteristically showed a larger increase than that in patients with localized disease. Finally, the groups showing an increase in the AAPC over the entire period were men, those under the age of 20 years, those in their 20s, those in their 30s, and those with regional disease. In contrast, in the SEER data, the SIR increased until 2015 and then began to decline steadily, regardless of sex and stage. Of note, the IR of thyroid cancer in young individuals (under 15) increased in the SEER data.
Until the early 2010s, the IR of thyroid cancer showed a very steep increase worldwide. Because concerns about overdiagnosis were raised in 2010, numerous studies on the IR trends of thyroid cancer have been steadily conducted in each geographic region. After the US Preventive Services Task Force announcement of its opposition to screening for asymptomatic thyroid cancer and changes in clinical guidelines, many regions began to show a decrease in the IR of thyroid cancer [16-18].
Korea experienced the highest thyroid cancer epidemic among other countries worldwide. The problem of the overdiagnosis of thyroid cancer has been examined and discussed by the medical community and the public [19-23]. Recent study reported the decrease of thyroid cancer incidence in Korea analyzing data from 1999 to 2016 [24]. In our study, we evaluated the more recent incidence data until 2018 and used the Korean mid-year resident registration population for age standardization. We demonstrate that the incidence of thyroid cancer was not decreased in men and younger age evident by positive AAPC during the entire study period in this subgroup. Recently, the incidence of thyroid cancer has been declining more slowly.
The gender disparity in the incidence of thyroid cancer is well known, and the differences have become even more pronounced recently as the incidence of the disease has increased [25,26]. To date, biological causes of gender-specific differences in thyroid cancer incidence are not exactly known. Moreover, it is believed that this is due to differences in healthcare use [25]. However, in this study, the APC of the IR increase until 2012 was steeper, and the decline after that was slower for men than for women. This phenomenon was also observed in another study [27].
Additionally, some recent studies reported the increasing incidence of childhood thyroid cancer in Korea [28,29] and worldwide [30-32]. In Korea, thyroid cancer is the most common cancer in both sexes among the adolescent and young adult populations (15 to 34 years), accounting for 50.8% of all cancers [17]. According to Park et al. [28], this might be accompanied by actual increases due to environmental factors, such as excessive iodine intake, exposure to medical radiation, and increased obesity prevalence, as well as the screening effect.
Stage-specific IRs increased for all SEER stages. Most studies revealed that the increase in the incidence of localized disease was the most obvious [33]. However, in this study, specifically, the increase in the IR of regional disease is the most remarkable. Furthermore, a study reported that Asians present with more advanced well-differentiated thyroid cancer than their White counterparts—a finding that is consistent with the findings of this study [34]. Studies to determine whether there are racial differences in the risk of developing thyroid cancer have been conducted before [35-38]. These differences could be contributed by not only access to medical care but also lifestyle, including diet, specifically iodine exposure; biological; and environmental factors.
On the other hand, we should consider whether there are differences in terms of the general public’s perception of the overdiagnosis of thyroid cancer between populations. Despite having an excellent prognosis, the diagnosis of thyroid cancer is a sentinel event in patients’ lives that has a profound and lasting psychosocial impact [39]. A major limitation is the inability to know prospectively with certainty which cancers are overdiagnosed [40]. Recently, in Korea, several studies on patient and public understanding of the issue of thyroid cancer overdiagnosis have been conducted [23,41,42]. According to these studies, a widespread lack of awareness of information on thyroid cancer overdiagnosis was noted, and even after receiving information, patients and the public were less concerned about overdiagnosis in deciding whether to undergo thyroid cancer screening.
This study has several limitations. First, we could not demonstrate any direct, specific cause of the trends in IRs. Second, we lacked data on tumor histology.
In conclusion, the incidence of thyroid cancer in Korea has decreased after 2012 and tended to increase again, and the increase was large in men and young individuals. Additionally, characteristically, there was a very distinct increase in the IR among patients with regional disease. The contribution of overdiagnosis to the increasing incidence of thyroid cancer could be substantial; however, a true increase in thyroid cancer incidence has not been ruled out. One of the greatest needs is developing biomarkers or tests that better distinguish indolent or very-slow-growing cancers from aggressive ones. Additionally, we need more profound education for the individuals under social and health system support, which can facilitate shared decisionmaking and informed consent. Lastly, continuously monitoring the trends in the epidemiology of thyroid cancer according to changes in the diagnostic nomenclature system and clinical guidelines and in-depth understanding of uncovered risk factors are necessary.
ACKNOWLEDGMENTS
This work was supported by the Korean Endocrine Society of EnM Research Award 2021 and a grant (2022IP0011) from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea.
REFERENCES
1. Li M, Dal Maso L, Vaccarella S. Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol. 2020; 8:468–70.

2. Kim J, Gosnell JE, Roman SA. Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol. 2020; 16:17–29.

3. Miranda-Filho A, Lortet-Tieulent J, Bray F, Cao B, Franceschi S, Vaccarella S, et al. Thyroid cancer incidence trends by histology in 25 countries: a population-based study. Lancet Diabetes Endocrinol. 2021; 9:225–34.

4. Kitahara CM, Pfeiffer RM, Sosa JA, Shiels MS. Impact of overweight and obesity on US papillary thyroid cancer incidence trends (1995-2015). J Natl Cancer Inst. 2020; 112:810–7.

5. Sanabria A, Kowalski LP, Shah JP, Nixon IJ, Angelos P, Williams MD, et al. Growing incidence of thyroid carcinoma in recent years: factors underlying overdiagnosis. Head Neck. 2018; 40:855–66.

6. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133.

7. Pereira M, Williams VL, Hallanger Johnson J, Valderrabano P. Thyroid cancer incidence trends in the United States: association with changes in professional guideline recommendations. Thyroid. 2020; 30:1132–40.

8. Pizzato M, Li M, Vignat J, Laversanne M, Singh D, La Vecchia C, et al. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol. 2022; 10:264–72.

9. Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015; 47:127–41.

10. Ahn HS, Welch HG. South Korea’s thyroid-cancer “epidemic”: turning the tide. N Engl J Med. 2015; 373:2389–90.

11. Davies L, Petitti DB, Martin L, Woo M, Lin JS. Defining, estimating, and communicating overdiagnosis in cancer screening. Ann Intern Med. 2018; 169:36–43.

12. Welch HG, Kramer BS, Black WC. Epidemiologic signatures in cancer. N Engl J Med. 2019; 381:1378–86.

13. Kitahara CM, Sosa JA. Understanding the ever-changing incidence of thyroid cancer. Nat Rev Endocrinol. 2020; 16:617–8.

14. Surveillance, Epidemiology, and End Results. SEER*Explorer: an interactive website for SEER cancer statistics [Internet]. Bethesda: Surveillance Research Program, National Cancer Institute;2022. [cited 2022 Sep 9]. Available from: https://seer.cancer.gov/statistics-network/explorer/.
15. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000; 19:335–51.

16. Lee M, Powers AE, Morris LGT, Marti JL. Reversal in thyroid cancer incidence trends in the United States, 2000- 2017. Thyroid. 2020; 30:1226–7.

17. Hong S, Won YJ, Lee JJ, Jung KW, Kong HJ, Im JS, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2018. Cancer Res Treat. 2021; 53:301–15.

18. Ellison LF, Bushnik T. Changing trends in thyroid cancer incidence in Canada: a histologic examination, 1992 to 2016. Health Rep. 2020; 31:15–25.
19. Ahn HS, Kim HJ, Welch HG. Korea’s thyroid-cancer “epidemic”: screening and overdiagnosis. N Engl J Med. 2014; 371:1765–7.

20. Park S, Oh CM, Cho H, Lee JY, Jung KW, Jun JK, et al. Association between screening and the thyroid cancer “epidemic” in South Korea: evidence from a nationwide study. BMJ. 2016; 355:i5745.

21. Han MA, Choi KS, Lee HY, Kim Y, Jun JK, Park EC. Current status of thyroid cancer screening in Korea: results from a nationwide interview survey. Asian Pac J Cancer Prev. 2011; 12:1657–63.
22. Yi KH. The revised 2016 Korean Thyroid Association guidelines for thyroid nodules and cancers: differences from the 2015 American Thyroid Association guidelines. Endocrinol Metab (Seoul). 2016; 31:373–8.

23. Lee S, Lee YY, Yoon HJ, Choi E, Suh M, Park B, et al. Responses to overdiagnosis in thyroid cancer screening among Korean women. Cancer Res Treat. 2016; 48:883–91.

24. Oh CM, Lim J, Jung YS, Kim Y, Jung KW, Hong S, et al. Decreasing trends in thyroid cancer incidence in South Korea: what happened in South Korea? Cancer Med. 2021; 10:4087–96.

25. LeClair K, Bell KJL, Furuya-Kanamori L, Doi SA, Francis DO, Davies L. Evaluation of gender inequity in thyroid cancer diagnosis: differences by sex in US thyroid cancer incidence compared with a meta-analysis of subclinical thyroid cancer rates at autopsy. JAMA Intern Med. 2021; 181:1351–8.

26. Yao R, Chiu CG, Strugnell SS, Gill S, Wiseman SM. Gender differences in thyroid cancer: a critical review. Expert Rev Endocrinol Metab. 2011; 6:215–43.

27. Zhai M, Zhang D, Long J, Gong Y, Ye F, Liu S, et al. The global burden of thyroid cancer and its attributable risk factor in 195 countries and territories: a systematic analysis for the Global Burden of Disease Study. Cancer Med. 2021; 10:4542–54.

28. Park J, Park H, Kim TH, Kim SW, Jang HW, Chung JH. Trends in childhood thyroid cancer incidence in Korea and its potential risk factors. Front Endocrinol (Lausanne). 2021; 12:681148.

29. Lee YA, Yun HR, Lee J, Moon H, Shin CH, Kim SG, et al. Trends in pediatric thyroid cancer incidence, treatment, and clinical course in Korea during 2004-2016: a nationwide population-based study. Thyroid. 2021; 31:902–11.

30. Dermody S, Walls A, Harley EH Jr. Pediatric thyroid cancer: an update from the SEER database 2007-2012. Int J Pediatr Otorhinolaryngol. 2016; 89:121–6.

31. Qian ZJ, Jin MC, Meister KD, Megwalu UC. Pediatric thyroid cancer incidence and mortality trends in the United States, 1973-2013. JAMA Otolaryngol Head Neck Surg. 2019; 145:617–23.

32. Vaccarella S, Lortet-Tieulent J, Colombet M, Davies L, Stiller CA, Schuz J, et al. Global patterns and trends in incidence and mortality of thyroid cancer in children and adolescents: a population-based study. Lancet Diabetes Endocrinol. 2021; 9:144–52.

33. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. 2017; 317:1338–48.

34. Harari A, Li N, Yeh MW. Racial and socioeconomic disparities in presentation and outcomes of well-differentiated thyroid cancer. J Clin Endocrinol Metab. 2014; 99:133–41.

35. Haselkorn T, Stewart SL, Horn-Ross PL. Why are thyroid cancer rates so high in southeast Asian women living in the United States?: the bay area thyroid cancer study. Cancer Epidemiol Biomarkers Prev. 2003; 12:144–50.
36. Horn-Ross PL, McClure LA, Chang ET, Clarke CA, Keegan TH, Rull RP, et al. Papillary thyroid cancer incidence rates vary significantly by birthplace in Asian American women. Cancer Causes Control. 2011; 22:479–85.

37. Magreni A, Bann DV, Schubart JR, Goldenberg D. The effects of race and ethnicity on thyroid cancer incidence. JAMA Otolaryngol Head Neck Surg. 2015; 141:319–23.

38. Lee AW, Mendoza RA, Aman S, Hsu R, Liu L. Thyroid cancer incidence disparities among ethnic Asian American populations, 1990-2014. Ann Epidemiol. 2022; 66:28–36.

39. Jensen CB, Pitt SC. Patient perception of receiving a thyroid cancer diagnosis. Curr Opin Endocrinol Diabetes Obes. 2021; 28:533–9.

40. Davies L, Hendrickson CD, Hanson GS. Experience of US patients who self-identify as having an overdiagnosed thyroid cancer: a qualitative analysis. JAMA Otolaryngol Head Neck Surg. 2017; 143:663–9.

41. Lee B, Park JY, Shin HY, Park SH, Choi EB, Yoo J, et al. What do Korean women know and want to know about thyroid cancer?: a qualitative study. Asian Pac J Cancer Prev. 2016; 17:2901–7.
Fig. 1.
Standardized incidence rates (SIRs) of thyroid cancer (A) in Korea, and annual percent change of the SIR in Korea in (B) total, (C) male, and (D) female. (E) The SIR in the Surveillance, Epidemiology, and End Results Program (SEER) data. APC, annual percent change; IR, incidence rate. aIndicates that the APC is significantly different from zero at the alpha=0.05 level.
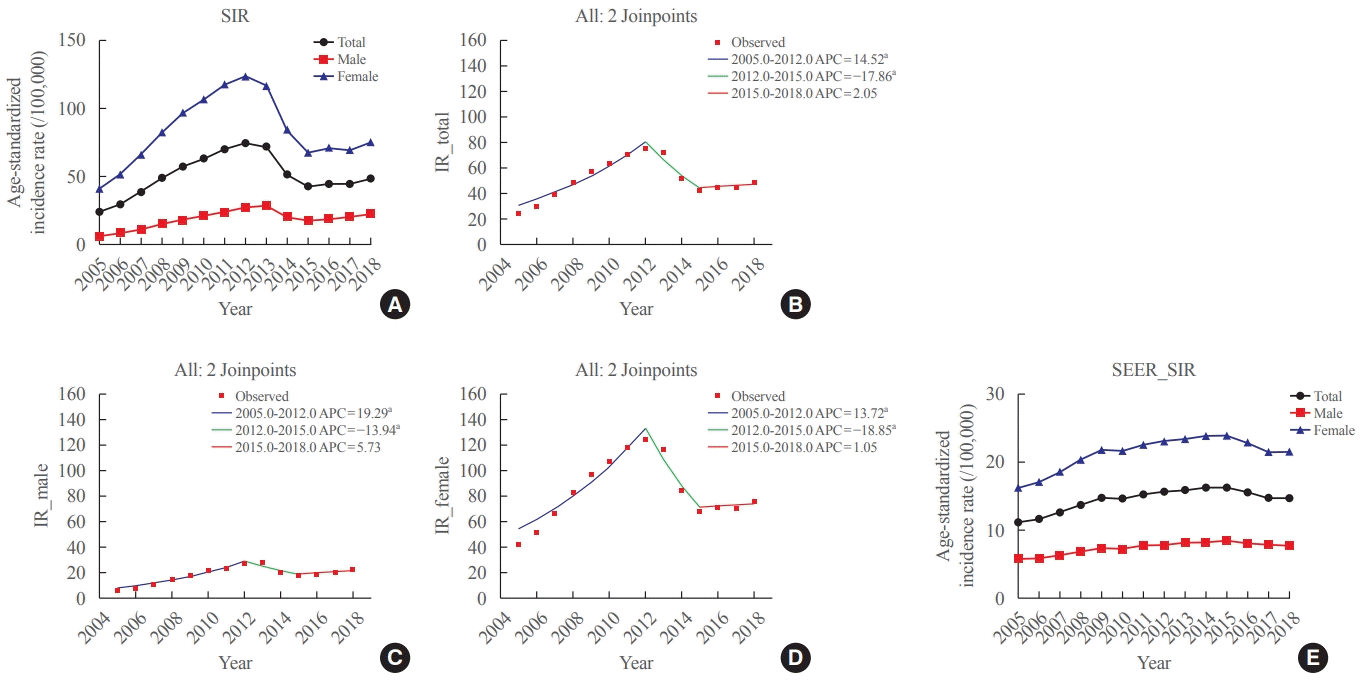
Fig. 2.
Age-specific thyroid cancer incidence rates (A) in Korea, and annual percent change of the age-specific incidence in Korea by age groups: (B) <20, (C) 20–29, (D) 30–39, (E) 40–49, (F) 50–59, (G) 60–69, and (H) ≥70 years. (I) Age-specific thyroid cancer incidence rates in the Surveillance, Epidemiology, and End Results Program (SEER) data. APC, annual percent change; IR, incidence rate. aIndicates that the APC is significantly different from zero at the alpha=0.05 level.
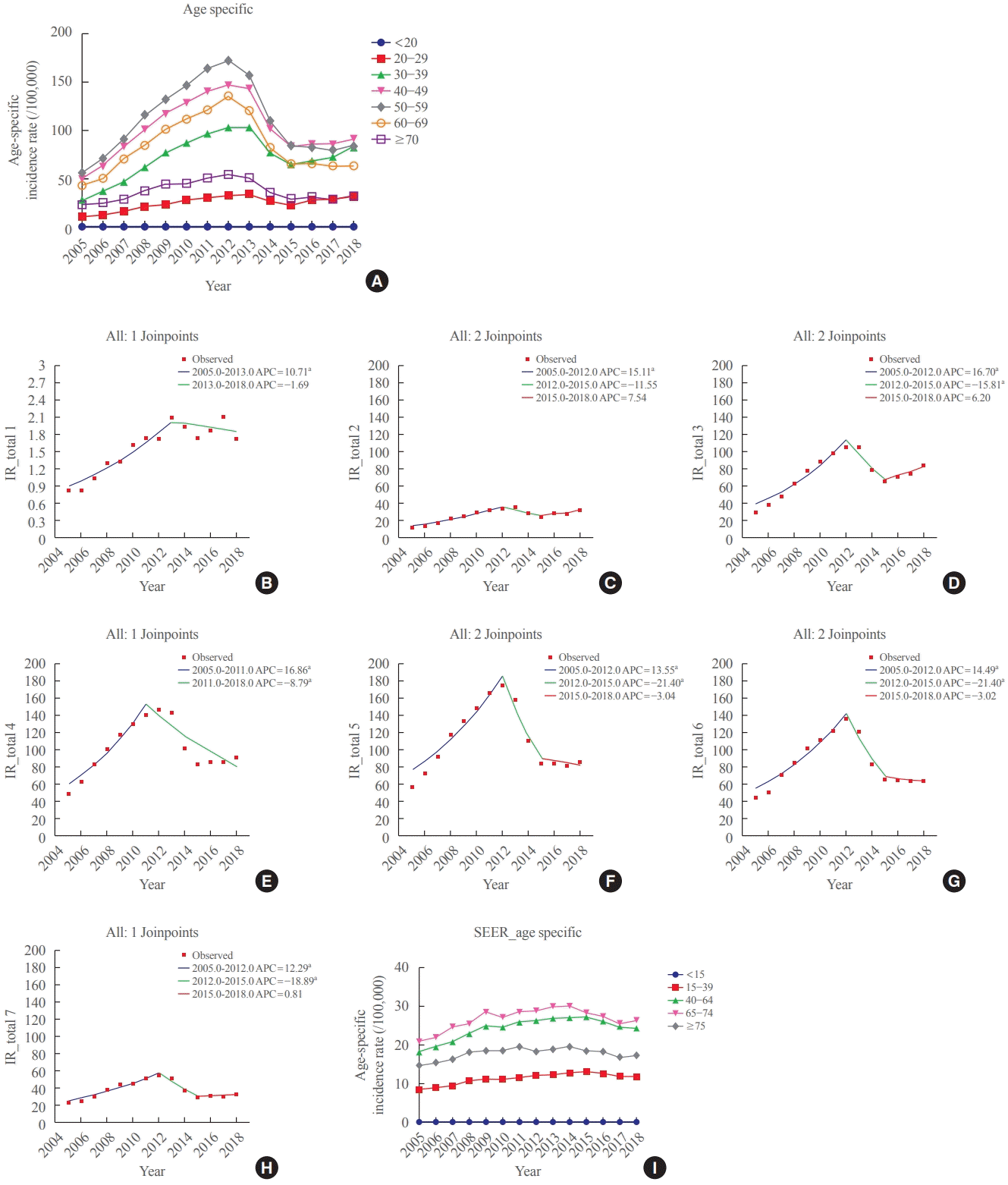
Fig. 3.
Stage-specific thyroid cancer incidence rates (A) in Korea, and annual percent change of the stage-specific incidence in Korea according to the stage groups: (B) regional, (C) localized, and (D) distant. (E) Stage-specific thyroid cancer incidence rates in the Surveillance, Epidemiology, and End Results Program (SEER) data. APC, annual percent change; IR, incidence rate. aIndicates that the APC is significantly different from zero at the alpha=0.05 level.
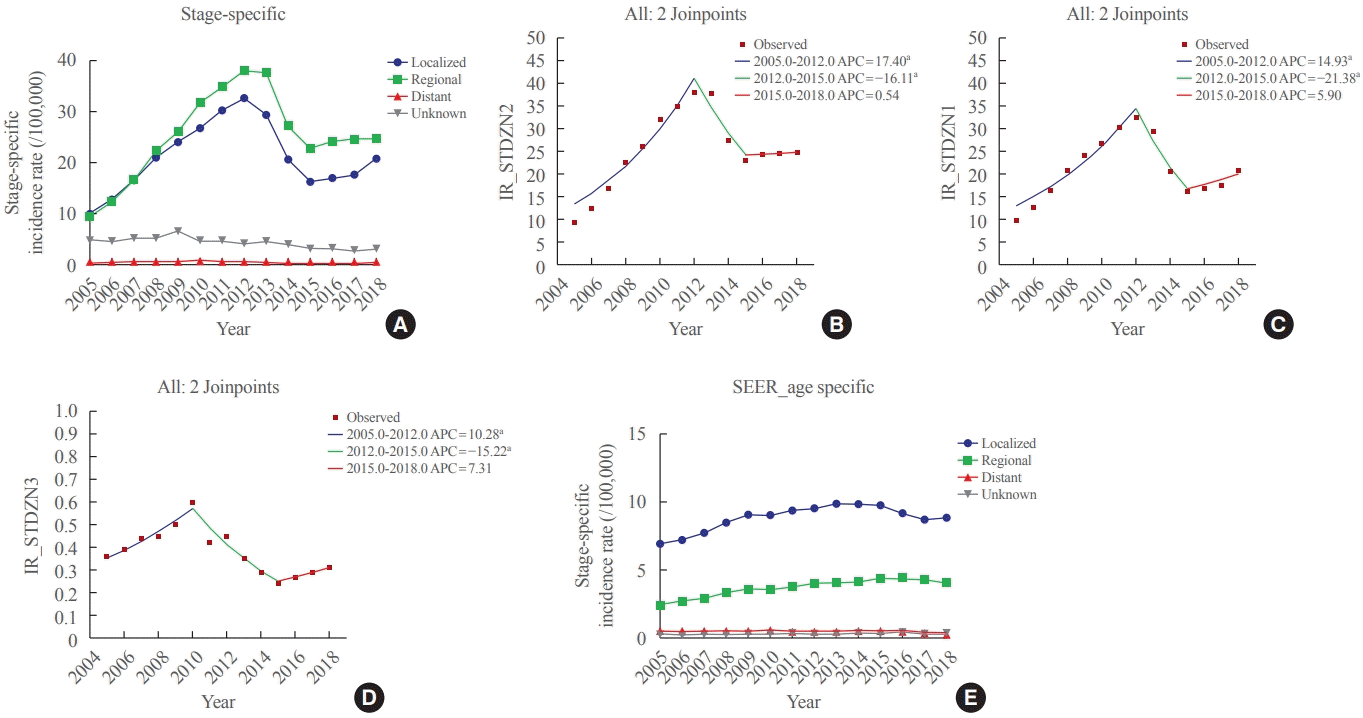
Table 1.
Age-Standardized, Age-Specific, and Stage-Specific Incidence Rates of Thyroid Cancer from 2005 to 2018 in Korea
Table 2.
Trends in Thyroid Cancer Incidence in Korea between 2005 and 2018 Using a Joinpoint Regression Model
|
Trend 1 |
Trend 2 |
Trend 3 |
AAPC (2005–2018) | |||||
|---|---|---|---|---|---|---|---|---|
| Years | APC (95% CI) | Years | APC (95% CI) | Years | APC (95% CI) | |||
| Age-standardized rate | ||||||||
| Total | 2005–2012 | 14.5 (10.5–18.7)a | 2012-2015 | −17.9 (−30.2 to −3.4)a | 2015–2018 | 2.1 (−9.1 to 14.6) | 3.3 (–0.8 to 7.5) | |
| Men | 2005–2012 | 19.3 (14.7–24.1)a | 2012-2015 | −13.9 (−24.0 to −2.6)a | 2015–2018 | 5.7 (−1.9 to 13.9) | 7.6 (4.2 to 11.1)a | |
| Women | 2005–2012 | 13.7 (9.7–17.8)a | 2012-2015 | −18.8 (−31.8 to −3.4)a | 2015–2018 | 1.1 (−11.3 to 15.1) | 2.4 (–1.9 to 6.9) | |
| Age groups, yr | ||||||||
| <20 | 2005–2013 | 10.7 (5.9–15.8)a | 2013–2018 | −1.7 (−6.5 to 3.3) | 5.8 (2.7 to 8.9)a | |||
| 20–29 | 2005–2012 | 15.1 (10.9–19.5)a | 2012–2015 | −11.5 (−23.5 to 2.3) | 2015–2018 | 7.5 (−0.2 to 15.9) | 6.6 (3.0 to 10.3)a | |
| 30–39 | 2005–2012 | 16.7 (12.0–21.6)a | 2012–2015 | −15.8 (−28.1 to −1.4)a | 2015–2018 | 6.2 (−3.9 to 17.3) | 5.9 (1.9 to 10.1)a | |
| 40–49 | 2005–2011 | 16.9 (7.2–27.3)a | 2011–2018 | −8.8 (−12.7 to −4.7)a | 2.3 (–1.7 to 6.4) | |||
| 50–59 | 2005–2012 | 13.5 (9.9–17.3)a | 2012–2015 | −21.4 (−32.5 to −8.5)a | 2015–2018 | −3.0 (−15.3 to 11.0) | 0.6 (–3.4 to 4.7) | |
| 60–69 | 2005–2012 | 14.5 (10.1–19.0)a | 2012–2015 | −21.4 (−34.8 to −5.3)a | 2015–2018 | −3.0 (−16.6 to 12.8) | 1.0 (–3.7 to 5.9) | |
| ≥70 | 2005–2012 | 12.3 (8.3–16.5)a | 2012–2015 | −18.9 (−31.7 to −3.7)a | 2015–2018 | 0.8 (−10.6 to 13.7) | 1.6 (–2.6 to 6.0) | |
| Summary stage groups | ||||||||
| Localized | 2005–2012 | 14.9 (11.1–18.9)a | 2012–2015 | −21.4 (−33.7 to −6.7)a | 2015–2018 | 5.9 (−6.2 to 19.5) | 3.3 (–0.9 to 7.7) | |
| Regional | 2005–2012 | 17.4 (12.6–22.4)a | 2012–2015 | −16.1 (−27.9 to −2.4)a | 2015–2018 | 0.5 (−9.7 to 11.9) | 4.8 (0.8 – 9.0)a | |
| Distant | 2005–2010 | 10.3 (4.8–16.0)a | 2010–2015 | −15.2 (−22.9 to −6.8)a | 2015–2018 | 7.3 (−12.2 to 31.1) | –1.0 (–5.8 to 4.1) | |
| Unknown | 2005–2009 | 7.9 (1.6–14.5)a | 2009–2018 | −8.8 (−11.3 to −6.2)a | –4.0 (–6.2 to –1.7)a | |||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download