Abstract
Objective
Stent retrieval thrombectomy has recently been the standard treatment for acute ischemic stroke with large artery occlusion. However, the development of catheters for suction thrombectomy has recently led to results comparable to that of stent retrieval thrombectomy (SRT). This study aimed to analyze the safety and efficacy of forced suction thrombectomy (FST) using the SOFIA Plus (MicroVention Terumo, Tustin, CA, USA) device.
Methods
We included patients with acute ischemic stroke who underwent FST using the SOFIA Plus device at our institution. Medical records and angiographic data were reviewed, and the results of this study were compared with those of other FST studies.
Results
A total of 35 patients were included in this study. The occlusion sites were the internal carotid artery terminal (4), M1 segment (20), and posterior circulation (11). Of the 35 patients, FST was performed in only 21 (60%) patients, and the remaining 14 (40%) patients underwent SRT and FST. In all cases, the recanalization rate was 100%, and the average time from groin puncture to recanalization was 21±4.94 min. In particular, the average time required to reach the SOFIA Plus lesions from the groin puncture was 10.44±5.06 min and about 67% of the FST patients were recanalized at the first attempt. Three-months modified Rankin Scale (mRS) score of ≤2 was observed in 52% of the patients.
Several prospective randomized clinical trials in 2015 demonstrated the effectiveness of mechanical thrombectomy in the treatment of acute ischemic stroke caused by large artery occlusion [1,2,4,10,21]. Retrievable stents are mainly used in randomized clinical trials. Thrombectomy devices are typically divided into stent and non-stent devices, and thrombectomy using Merci, a non-stent device, was reported to be less effective than stent retrieval thrombectomy (SRT) [18,22].
However, good results have recently been reported for suction thrombectomy using a large-bore catheter. Suction thrombectomy has several names, but the methods are similar [7,8,13,14,16]. Mechanical thrombectomy performed with a suction catheter is referred to as forced suction thrombectomy (FST). An FST using large bore and highly trackable catheters, among non-stent devices, has yielded good results [6,9,13,14,16,19,24]. Additionally, the recanalization rate of FST is reportedly higher than that of SRT [16]. Catheters must have high trackability to easily reach the lesion and a sufficiently large bore to engage the thrombus [8,16]. The SOFIA Plus (MicroVention Terumo, Tustin, CA, USA) has the largest bore of 6 Fr distal access catheter. Additionally, the proximal part reinforces more support, and the distal part is made very flexible to improve trackability because the wire braid is different for each part of the SOFIA Plus [28]. We aimed to retrospectively evaluate the clinical results of FST using the SOFIA Plus device, which has all required characteristics.
This study was approved by the institutional review board. The requirement for written informed consent to participate in the study was waived. All methods were performed in accordance with the relevant guidelines and regulations by including a statement. The medical records of all patients with acute ischemic stroke who underwent mechanical thrombectomy at our institution from November 2017 to July 2019 were reviewed.
The FST was performed in patients with acute ischemic stroke due to large-artery occlusion. Patients who underwent thrombectomy were initially diagnosed radiologically with a diffusion-perfusion mismatch on radiological examination. Patients with acute ischemic stroke of the anterior circulation within 6 h of symptom onset and within 24 h of symptom onset in acute ischemic stroke of the posterior circulation were included. Within 4.5 h of symptoms onset, intravenous tissue plasminogen was used.
Patients in whom a 6 Fr SOFIA Plus was used as the forced suction thrombectomy device were enrolled, and those in whom another catheter or stent retrieval thrombectomy was used as the primary treatment were excluded. Patients who underwent intracranial or carotid artery stenting were also excluded.
All procedures were performed under local anesthesia by a neurointerventionist. A double coaxial system, combining a 6 Fr shuttle (Cook Medical, Bloomington, IN, USA) and a 125 cm diagnostic angiocatheter (Cook Medical, Bloomington, IN, USA) was placed in the proximal portion of the parent artery. An initial angiogram was used to determine the occlusion site and collateral vessels. The SOFIA Plus was placed proximal to the occluded site through a double coaxial microcatheter (Excelsior XT-27, Stryker, Fremont, California, USA), with the microcatheter placed in the distal branch through the occluded site to accelerate the passage of SOFIA Plus. The SOFIA Plus was pushed towards the occlusion to ensure more blood clots into the catheter, and a negative force was applied by connecting a 50 mL syringe. If it was confirmed that there was no backflow of blood through the syringe, the catheter was maintained for 3 min to inactivate the thrombus. After the 3 min, the shuttle was stopped, heparinized saline flushing was performed, and the SOFIA Plus was slowly removed while checking backflow of blood. When blood backflow was observed during the catheter removal, approximately 20 mL of blood was removed from the SOFIA Plus at that location. The roadmap image was used to assess recanalization; if recanalization was not observed, the procedure was repeated. When the SOFIA Plus was completely removed without blood backflow, the presence of a thrombus was confirmed in the tip or inner lumen of the SOFIA Plus. Stent retrieval thrombectomy was subsequently performed if recanalization was not achieved after multiple attempts or distal embolic occlusion occurred. The removal of the distal M2 embolism was attempted using SOFIA Plus.
The recanalization rate was determined by two neurointerventionists using digital subtraction angiography after thrombectomy and thrombolysis in cerebral ischemia (TICI) scale was used [29]. Successful recanalization was defined as a TICI ≥2b. Recanalization time was measured from the femoral artery puncture to confirm recanalization. Modified Rankin Scale (mRS) [26] was checked at admission and three months later, and a favorable outcome was defined as an mRS ≤2. Procedure-related intracranial hemorrhage was confirmed using brain computed tomography performed immediately after the thrombectomy. Mortality was determined within 90 days of admission.
A total of 81 patients underwent thrombectomy, and the SOFIA Plus was used in 35 patients. There were 21 FST patients and 14 combined with SRT. The mean age was 68.6±14.2 years and the sex ratio included more males (22/35). There were 24 clots in the anterior circulation (ICA terminal 4, M1 segment 20) and 11 in the posterior circulation. The time from symptom onset to admission was 283 min, and from diagnosis of infarction to arterial puncture was 97 min. Intravenous tissue-plasminogen activator was used in nine patients. The patients’ demographic characteristics details are summarized in Table 1.
The average time from groin puncture to catheter to lesion was 10.44±5.06 min. The recanalization time from groin puncture was 17.31±7.44 min in FST alone, 32.83±21.21 min in combined SRT, and 21.04±14.53 min in total. The average time required to reach the SOFIA Plus lesions from the groin puncture was 10.44±5.06 min. The average number of attempts in the FST alone was 1.7±1.39 times. In the combined SRT, they switched to SRT after 5.4±2.48 FST attempts. In particular, FST alone was recanalized on the first attempt in 14 patients. The total recanalization rates of TICI ≥2b and TICI ≥3 were 100% and 77.15%, respectively. The recanalization rate with TICI 3 was 80.95% for FST alone and 71.42% for combined SRT.
Distal embolic occlusion occurred in five patients. Four patients were recanalized using the SOFIA Plus, and one patient was recanalized using the combined SRT. Procedural-related hemorrhage occurred in four subarachnoid hemorrhages post-thrombectomy brain tomography, but the amount was small and there was no clinical morbidity.
The initial National Institutes of Health Stroke Scale score was 12.06±4.45 and it decreased to 7.57±5.48 at discharge. On average, the initial mRS score was 3.84±1.08, and 2.48±2.04 at 90 days after admission. Nineteen (54.28%) patients had an mRS score of ≤2 at 90 days after their admission. Two patients died 90 days after admission, and the cause of death was not directly related to stroke.
The results of forced suction thrombectomy with the Sofia Plus are summarized in Table 2.
The treatment of acute ischemic stroke due to large artery occlusion has been mainly conservative for a long time, but recent prospective randomized clinical trials reported in 2015 demonstrated superior clinical effects of thrombectomy over conventional medical treatment [1,2,4,10,21]. All these studies performed thrombectomy using a retrievable stent, and the use of the stent was suggested as a guideline for the treatment of acute ischemic stroke with large artery occlusion using thrombectomy [1,2,4,10,15,21]. However, suction revascularization has long been used in peripheral vessels, and a good effect of suction thrombectomy on intracranial large artery occlusion has been reported [8,9,13,14,16,19,24,28].
Advantages of suction thrombectomy over SRT include removal without thrombus fragmentation, faster combination with SRT, and less intimal injury [3,20]. However, it is difficult to reach a large-bore catheter. This disadvantage has been a major problem in the development of distal-access catheters. Previous reports on suction thrombectomy mostly resulted from the Penumbra system, but recently, thrombectomy with various distal access catheters was also reported [7,17,19,23]. To date, there are still reports of SOFIA 5 Fr use [11,19,23].
The first requirement for a successful suction thrombectomy is a large-bore catheter [14,16,24,25]. In comparison with the 6 Fr distal access catheter, the SOFIA Plus has the largest bore (0.070 inch) and suction force at the distal tip of the catheter (21.32 g) [5]. The aspiration rate of the SOFIA Plus is 285 mL/min, which is not significantly different from that of Penumbra ACE 0.68 [5]. However, the suction force of the catheter is important because blood flow becomes zero when the blood clot sufficiently engages the catheter [12]. The second factor was trackability, of which its determination is subjective. During FST, it is difficult for the suction catheter to pass through the siphon area where the curvature of the internal carotid artery is the largest. To solve this problem, the microcatheter and microwire should be navigated to the remote distal branch of the occlusion part, or the retrieval stent can be deployed to the distal portion of the occluded site. The suction catheter can be placed in the occluded site along the wire of the stent. However, this process is time-consuming and can damage blood vessels in the distal artery of the occluded site. In our results, the SOFIA Plus’s trackability suggested that the catheter rapidly and easily reached the lesion within 10.44±5.06 min of average time from the groin puncture to when the catheter gets to lesion. The trackability of SOFIA Plus for severely tortuous proximal vessels and distal embolic occlusion was relatively good (Fig. 1A). Jang et al. [6] performed an FST using Penumbra ACE for internal carotid artery (ICA) occlusion and reported that it took approximately 20 min on average to reach the occlusion site. However, because this was a lesion limited to the ICA, it cannot be regarded as better than our results for most M1 occlusions. In our case, distal embolic occlusion occurred after FST in four patients, and all of them were recanalized using the SOFIA plus. Although it depends on arterial diameter, the FST can be performed even for M2 and M3 using the SOFIA Plus (Fig. 1B). However, we believe that the use of a balloon-guiding catheter is necessary to prevent distal embolisms. Table 3 summarizes the results of the comparison with FST using the other devices.
Forced suction thrombectomy using a large-bore catheter has shown faster recanalization and better clinical results than a small internal catheter [14,16,24]. In previous studies, the recanalization rate of a TICI scale ≥2b in FST was reported to be 53–88% [7,13,14,19,24]. However, our results for FST alone showed a high recanalization rate of 100% for TICI ≥2b, and especially, 80.95% for TICI ≥3. In other reports, the time from groin puncture to recanalization reportedly varied from between 34-73 min, but our result was 21.04±14.53 min, 13 min shorter than the best result, and the FST alone was shortened by 24 min. In various reports, mRS ≤2 at 90 days after the onset of symptoms varied between 40-79% [7,9,13,14,24,25], and our results were similar at 54.28%. Rapid recanalization is important for the outcome of acute ischemic stroke due to large artery occlusion; however, the presence of abundant collateral flow is the most important factor. Abundant collateral flow prolongs the window period of ischemic injury. Since the degree of collateral flow varies from person to person, fast recanalization is one of the factors that can make good prognosis possible.
In Jeon’s meta-analysis, if FST was selected first, the probability of using an additional device was higher than that of SRT. The combination of FST and SRT showed a high recanalization rate, but their outcome varied [11,15,16,27]. The transition from SRT to FST is time consuming, but vice versa, it is not. Since the combination of FST and SRT can increase medical costs, the device selected for recanalization should be selected by the neurointerventionist who is most confident of the device. In our study, only 40% of the patients used a second device.
The limitation of this study was that it did not provide an objective indicator of trackability, and there was a tendency for data due to the majority of M1 occlusions. We also needed to statistically compare the results with those of other devices.
Since the outcome of patients with large artery occlusion stroke is the time of rapid recanalization from symptom onset, rapid systematic brain imaging studies and rapid recanalization are required for patients with indications. An appropriate thrombectomy device should be selected for rapid recanalization. Thus, the SOFIA Plus is considered a suitable FST device for patients with large-artery occlusion stroke.
REFERENCES
1. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015; Jan. 372(1):11–20.
2. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015; Mar. 372(11):1009–18.

3. Gory B, Bresson D, Kessler I, Perrin M, Guillaudeau A, Durand K, et al. Histopathologic evaluation of arterial wall response to 5 neurovascular mechanical thrombectomy devices in a swine model. AJNR Am J Neuroradiol. 2013; Nov-Dec. 34(11):2192–8.

4. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015; Mar. 372(11):1019–30.

5. Hu YC, Stiefel MF. Force and aspiration analysis of the ADAPT technique in acute ischemic stroke treatment. J Neurointerv Surg. 2016; Mar. 8(3):244–6.

6. Jang HG, Park JS, Lee JM, Kwak HS. Initial experience of ACE68 reperfusion catheter in patients with acute ischemic stroke related to internal carotid artery occlusion. J Korean Neurosurg Soc. 2019; Sep. 62(5):545–50.

7. Jankowitz B, Grandhi R, Horev A, Aghaebrahim A, Jadhav A, Linares G, et al. Primary manual aspiration thrombectomy (MAT) for acute ischemic stroke: safety, feasibility and outcomes in 112 consecutive patients. J Neurointerv Surg. 2015; Jan. 7(1):27–31.

8. Jeon JP, Kim SE, Kim CH. Primary suction thrombectomy for acute ischemic stroke: a meta-analysis of the current literature. Clin Neurol Neurosurg. 2017; Dec. 163:46–52.

9. John S, Hussain MS, Toth G, Bain M, Uchino K, Hui FK. Initial experience using the 5MAX™ ACE reperfusion catheter in intra-arterial therapy for acute ischemic stroke. J Cerebrovasc Endovasc Neurosurg. 2014; Dec. 16(4):350–7.

10. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015; Jun. 372(24):2296–306.

11. Kabbasch C, Möhlenbruch M, Stampfl S, Mpotsaris A, Behme D, Liebig T. First-line lesional aspiration in acute stroke thrombectomy using a novel intermediate catheter: initial experiences with the SOFIA. Interv Neuroradiol. 2016; Jun. 22(3):333–9.

12. Kallmes DF, Sadasivan C, Fiorella D. The truth and fiction in aspiration physics: may the forces be with you. J Neurointerv Surg. 2018; Nov. 10(11):1029–30.

13. Kang DH, Hwang YH, Kim YS, Park J, Kwon O, Jung C. Direct thrombus retrieval using the reperfusion catheter of the penumbra system: forced-suction thrombectomy in acute ischemic stroke. AJNR Am J Neuroradiol. 2011; Feb. 32(2):283–7.

14. Kim YS, Kwak HS, Chung GH, Hwang SB. Manual aspiration thrombectomy using the Penumbra catheter in patients with acute M1 occlusion: a single-center study. Interv Neuroradiol. 2015; Dec. 21(6):694–9.

15. Kowoll A, Weber A, Mpotsaris A, Behme D, Weber W. Direct aspiration first pass technique for the treatment of acute ischemic stroke: initial experience at a European stroke center. J Neurointerv Surg. 2016; Mar. 8(3):230–4.

16. Lapergue B, Blanc R, Guedin P, Decroix JP, Labreuche J, Preda C, et al. A direct aspiration, first pass technique (ADAPT) versus stent retrievers for acute stroke therapy: an observational comparative study. AJNR Am J Neuroradiol. 2016; Oct. 37(10):1860–5.

17. Lee HC, Kang DH, Hwang YH, Kim YS, Kim YW. Forced arterial suction thrombectomy using distal access catheter in acute ischemic stroke. Neurointervention. 2017; Mar. 12(1):45–9.

18. Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015; Oct. 46(10):3020–35.

19. Romano DG, Cioni S, Vinci SL, Pero G, Comelli C, Comai A, et al. Thromboaspiration technique as first approach for endovascular treatment of acute ischemic stroke: initial experience at nine Italian stroke centers. J Neurointerv Surg. 2017; Jan. 9(1):6–10.

20. Sakai N, Ota S, Matsumoto Y, Kondo R, Satow T, Kubo M, et al. Efficacy and safety of REVIVE SE thrombectomy device for acute ischemic stroke: river Japan (reperfuse ischemic vessels with endovascular recanalization device in Japan). Neurol Med Chir (Tokyo). 2018; Apr. 58(4):164–72.

21. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015; Jun. 372(24):2285–95.

22. Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012; Oct. 380(9849):1241–9.

23. Stampfl S, Kabbasch C, Müller M, Mpotsaris A, Brockmann M, Liebig T, et al. Initial experience with a new distal intermediate and aspiration catheter in the treatment of acute ischemic stroke: clinical safety and efficacy. J Neurointerv Surg. 2016; Jul. 8(7):714–8.

24. Suzuki K, Aoki J, Sakamoto Y, Kanamaru T, Abe A, Suda S, et al. Efficiency of the penumbra 5MAX ACE reperfusion catheter in acute ischemic stroke patients. J Stroke Cerebrovasc Dis. 2016; Dec. 25(12):2981–6.

25. Turk AS, Frei D, Fiorella D, Mocco J, Baxter B, Siddiqui A, et al. ADAPT FAST study: a direct aspiration first pass technique for acute stroke thrombectomy. J Neurointerv Surg. 2014; 6:260–4.

26. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988; May. 19(5):604–7.

27. Vargas J, Spiotta A, Fargen K, Turner R, Chaudry I, Turk A. Long term experience using the ADAPT technique for the treatment of acute ischemic stroke. J Neurointerv Surg. 2017; May. 9(5):437–41.

Fig. 1.
(A) Fluoroscopic image of SOFIA Plus placed at the occluded lesion via tortuous proximal vessel. (B) Fluoroscopic image of SOFIA Plus placed to M3 for distal embolic occlusion at M3.
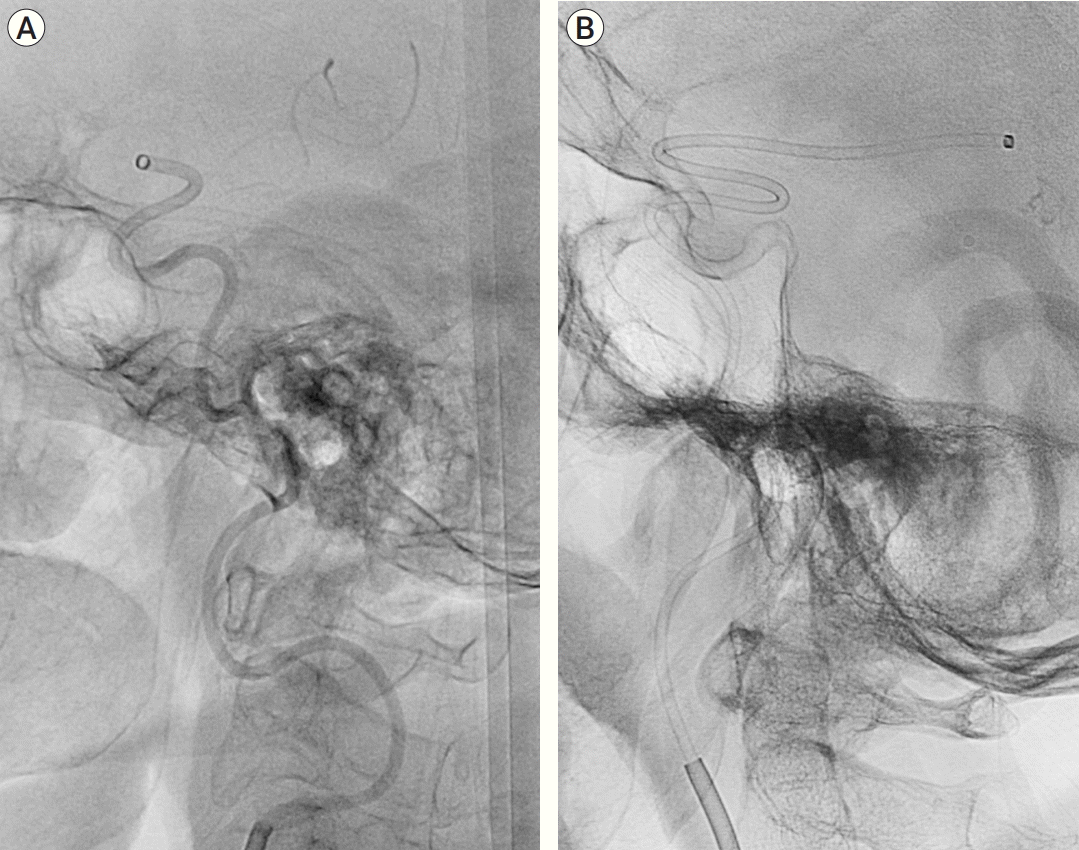
Table 1.
Baseline patient demographics and risk factors
Table 2.
Summary of results for forced suction thrombectomy with the Sofia Plus
Table 3.
Comparison of the present study with reported other suction thrombectomy results
| Case number | Occlusion site |
TICI ≥2b (%) |
Time from groin puncture to recanalization (minute) (FST alone vs combined SRT) | Recanalization on first attempt (%) | Complication (%) | mRS ≤2 (%) after 90 days | Device | ||
|---|---|---|---|---|---|---|---|---|---|
| FST | Combined SRT | ||||||||
| Kang DH et al. [13] (2011) | 22 | ICA 4 | 81.8 (Additional device: balloon 4.5, stent insertion 13.6) | 68 | No data | 31.8 (Hemorrhage) | 45.5 | Penumbra (3, 4Max) | |
| MCA 14 | |||||||||
| VBA 4 | |||||||||
| John S et al. [9] (2014) | 15 | ICA 8 | 20 | 73 | 46±30 | No data | 0 | 33 | Penumbra (ACE) |
| MCA 5 | 33 (TICI 3) | ||||||||
| VBA 2 | |||||||||
| Turk AS et al. [25] (2014) | 100 | ICA 23 | Overall 95 | 36.6±26.4 (31.6±23.3 vs 56.8±29.1) | 10 | 10 (Embolism) | 39 (missing case 23%) | Penumbra (ACE, 3~5Max) | |
| Tandem 11 | 5Max alone - 75 | ||||||||
| MCA 61 | 5Max ACE alone - 82 | 2 (Dissection) | |||||||
| VBA 5 | |||||||||
| Jankowitz B et al. [7] (2015) | 112 | ICA 21 | 86.6 (Additional device: SRT 28.5%, Merci 5.3%, IA tPA 2.6%) | 70 | No data | 17 | 46.1 | Penumbra (4 Max) | |
| MCA 79 | |||||||||
| VBA 12 | 044 DAC, Navien | ||||||||
| Tandem 21 | |||||||||
| Suzuki K et al. [24] (2016) | 24 | ICA 12 | 92 | 52 | No data | 7 | 79 | Penumbra (5Max) | |
| M1 12 | |||||||||
| Kim YS et al. [14] (2015) | 70 | M1 70 | 82.9 (TICI 3 28.6) | 91.4 | 44 | 56 | 18.3 (Hemorrhage) | 60 | Penumbra (4Max) |
| Stampfl S et al. [23] (2016) | 115 | ICA 28 | 86.9 | 73.1±59.9 | No data | 6.1 (Hemorrhage) | No data | 5 Fr SOFIA | |
| MCA 67 | |||||||||
| ACA 2 | 5.2 (Embolism) | ||||||||
| VBA 18 | |||||||||
| Romano DG et al. [19] (2017) | 152 | ICA 40 | 83.3 | 53.57 | 57.8 (44.67 vs 80.41) | No data | 7.8 (Hemorrhage) | 50.6 | 5Max ACE, 5Max Navien 0.58 5 Fr SOFIA |
| MCA 73 | |||||||||
| VBA 14 | 1.9 (Embolism) | ||||||||
| Tandem 25 | |||||||||
| Jang HG et al. [6] (2019) | 29 | ICA 29 | 83 | 20 | No data | 4 (Hemorrhage) | 48 | Penumbra ACE | |
| Present study | 35 | ICA 4 | 100 | 100 | 21.04±14.53 (17.31±7.44 vs 32.83±21.21) | 66.6 | 11.4 (Embolism) | 54.28 | SOFIA Plus |
| MCA 20 | 81.95 (TICI 3) | 71.43 (TICI 3) | 2.8 (Hemorrhage) | ||||||
| VBA 11 | |||||||||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download