Abstract
Objective
Non-aneurysmal spontaneous subarachnoid hemorrhage (NASAH) has a good prognosis, but its cause has not been clearly identified. In this study, we assessed the clinical and radiological features of NASAH and suggested an anatomical relationship between the basilar tip anatomy and NASAH.
Methods
From August 2013 to May 2020, 21 patients were diagnosed with NASAH at our institution. We evaluated the clinical features of NASAH. NASAH was classified into a perimesencephalic pattern and aneurysmal pattern according to the distribution of hemorrhage based on initial brain computed tomography. Digital subtraction angiography was used to classify the basilar tip anatomy into symmetric cranial fusion, symmetric caudal fusion, or asymmetric fusion types.
Results
Of the 21 patients, twenty patients had a good clinical outcome (modified Rankin Scale (mRS) 1–2; Glasgow Outcome Scale (GOS) 4–5). These patients showed improvement in mRS and Glasgow Coma Scale (GCS) at the last follow-up (P=.003 and P=.016, respectively). Eighteen patients with NASAH (85.7%) had the caudal fusion type, and only three patients with NASAH (14.3%) had the cranial fusion type. Seven patients with the perimesencephalic pattern (77.8%) had the caudal fusion type, and eleven patients with the aneurysmal pattern (91.7%) had the caudal fusion type.
The prevalence of spontaneous subarachnoid hemorrhage (SAH) is known to be 6-10 per 100,000 people [7,8,15]. The most common cause is rupture of a cerebral aneurysm, which accounts for more than 80% of all SAH cases [4]. There is also non-aneurysmal spontaneous SAH (NASAH), which has a better course and prognosis than SAH caused by aneurysmal rupture. NASAH can be divided into a perimesencephalic pattern and aneurysmal pattern according to the distribution of hemorrhage on brain computed tomography (CT) [2,4,8]. The cause of NASAH is not yet clear. Previous studies have suggested various hypotheses about the cause of NASAH such as occult aneurysm, vascular malformation, and intracranial arterial dissection [5,20,24,28]. However, no studies have investigated the association between basilar tip anatomy and NASAH. The purpose of this study was to evaluate the relationship between the basilar tip anatomy and NASAH.
From August 2013 to May 2020, 21 patients diagnosed with NASAH at our institution were enrolled, and we carefully reviewed the medical records and Picture Archiving and Communicating System (PCAS) retrospectively. Patients were diagnosed with spontaneous SAH through history taking and brain CT when they visited the emergency room. The patients had no head trauma and did not take antiplatelets or anticoagulation medications. On the same day, digital subtraction angiography (DSA) was performed to confirm the structural etiologies of SAH, such as cerebral aneurysms. In addition, brain magnetic resonance imaging (MRI) was used to evaluate whether there were any lesions that led to hemorrhage and occult in DSA, such as brain tumors or other cerebrovascular diseases. NASAH was divided into two types according to the distribution of hemorrhage on initial brain CT. A perimesencephalic pattern is defined as hemorrhage mainly localized anterior to the midbrain and pons and may extend to the basal cisterns, suprasellar cisterns, and up to proximal one-third of Sylvian fissure or anterior interhemispheric fissure. Small amounts of intraventricular hemorrhage may be accompanied, but there is no overt intraventricular hemorrhage [4,22]. On the other hand, cases in which hemorrhage is not limited to the perimesencephalic pattern are referred to as an aneurysmal pattern [4,22]. These two patterns are presented in Fig. 1A and 1B. This study was approved by the institutional review board with waiver of informed consent or exemption at our institution (approval no. 20-2021-44).
The patients were admitted to the intensive care unit and underwent supportive care in the acute period. Symptoms at the time of visit to the emergency room, initial Glasgow Coma Scale (GCS) [25], and modified Rankin Scale (mRS) [21] were evaluated, and epidemiologic factors such as hypertension, diabetes mellitus, dyslipidemia, heart disease, etc. were recorded. HuntHess grade (HH) [10] was evaluated based on initial neurological status and modified Fisher grade (mF) [6] was estimated based on initial brain CT. Outcomes of patients were assessed by mRS, GCS and Glasgow Outcome Scale (GOS) [11] at discharge, six months, 12 months, and at the last follow-up. A good outcome was defined as a clinical status with mRS 0–2, and GOS 4–5 at the last follow-up. Otherwise, the outcome was defined as poor.
The basilar tip morphologies were categorized according to whether the superior cerebellar artery (SCA) branched from the basilar artery (BA) or posterior cerebral artery (PCA). Cases with SCA that originated from BA were defined as the cranial fusion type, and those with SCA originating from PCA were defined as the caudal fusion type. The caudal fusion type had two subtypes, symmetric and asymmetric fusion, according to its symmetry [3,17]. The classification of the fusion type was based on the 3-dimensional reconstruction images from the rotational angiography of DSA. Representative cases for each type of basilar tip morphology are shown in Fig. 1C, 1D, and 1E. Among 21 patients, three patients (14.3%) with symmetrical cranial fusion, four patients (19%) with symmetric caudal fusion, and fourteen patients (66.7%) with asymmetric fusion were classified.
Continuous variables are presented as the mean±standard deviation (SD) along with the range of values. Categorical variables are presented as numbers and percentages of total cases. The Shapiro-Wilk test was used to assess normality. Student’s t-test was used to analyze differences in continuous variables that satisfied normality. On the other hand, when normality was not met, the Mann-Whitney U-test was used to analyze the difference between continuous variables. Fisher’s exact test was used to analyze differences in categorical variables. When analyzing the difference between the initial mRS and the last follow-up mRS, the Wilcoxon signedrank test was used. All statistical analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA) with a P-value <.05 considered statistically significant.
The basal characteristics and clinical features of the patients are presented in Table 1. A total of 21 NASAH patients were included; of these, nine patients had a perimesencephalic pattern, and twelve patients had an aneurysmal pattern. The mean age±SD (range) of the patients was 53.1±18.9 (21–76) and 60.5±8.0 (45–73) years in the perimesencephalic pattern group and aneurysmal pattern group, respectively. Male predominance was shown in the aneurysmal pattern (66.7%); on the other hand, the perimesencephalic pattern seemed to have a relatively even distribution according to sex. The most common initial symptom was headache (95.2% in total) in both groups, but there were some differences between the two groups regarding the frequencies of other symptoms. In the perimesencephalic pattern, nausea (44.4%) and vomiting (22.2%) were common, but in the aneurysmal pattern, altered mentality and vomiting (33.3% in each) were relatively frequent. The most common underlying disease in both groups was hypertension (42.9% in total). Despite differences in some variables, no statistically significant difference was revealed between the perimesencephalic pattern and aneurysmal pattern.
Clinical outcomes of NASAH are presented in Table 2. The mean follow-up duration of all patients was 15.7 months (range, 0–86 months). The symptoms and neurological conditions that most patients complained of when they first visited the emergency room improved. One patient in the aneurysmal pattern had hydrocephalus, but a ventriculoperitoneal shunt was not required. All perimesencephalic pattern patients (n=9) showed good clinical outcomes. Of the aneurysmal pattern patients (n=12), eleven patients had good clinical outcomes, and the other patient had a poor clinical outcome. However, there was no statistically significant difference in the clinical outcomes of the two groups. The changes in the initial mRS and the last follow-up mRS are presented in Fig. 2A. Excluding one patient with a poor clinical outcome in the aneurysmal pattern, all patients showed improvement in the last follow-up mRS compared to the initial mRS (P=.003). Changes in the initial GCS and the last follow-up GCS are also presented in Fig. 2B. In all patients, the last follow-up GCS was equal to or better than the initial GCS (P=.016).
Basilar tip anatomy and other radiologic findings are presented in Table 3. In NASAH, the basilar tip showed a caudal fusion type in 18 patients (85.7%) and an asymmetric fusion type in 14 patients (66.7%). In the perimesencephalic pattern, the caudal fusion type was found in 7 patients (77.8%), and the asymmetric fusion type was found in 6 patients (66.7%). Aneurysmal patterns also showed caudal fusion types in 11 patients (91.7%) and asymmetric fusion types in 8 patients (66.7%). However, the differences among the types of basilar tip anatomy between the patients with a perimesencephalic pattern and the patients with an aneurysmal pattern were not statistically significant. There was also an aneurysm in 5 patients (23.9%) that was accidentally discovered, which was not thought to be the cause of SAH because the aneurysms had benign shapes, small sizes, and did not match the distribution of SAH. Except for one patient with follow-up loss, four patients with unruptured aneurysms discovered by chance had a small aneurysm and were not subject to treatment but were under regular follow-up. Intracranial arterial stenosis was identified in two patients (9.5%).
Many hypotheses have been proposed regarding what could be the cause of NASAH, but the cause is still not clear. First, venous bleeding may cause NASAH. According to some reports, there was a tendency to develop NASAH in patients with primitive venous drainage [27,28]. Other papers reported that venous occlusion of the vein of Galen, jugular vein, and cavernous sinus might cause NASAH [14,16,23]. Second, the bleeding of NASAH may originate from the perforating artery. Numerous perforating arteries originated from the BA, PCA, and SCA [24]. Park et al. reported that 3 out of 13 NASAH patients had a rupture of tiny aneurysms at the origin of the mesencephalic perforator found in 3-dimensional rotational angiographic reconstruction images [20]. Other papers reported that leakage from ventriculostriate or thalamoperforating arterial bleeding could be the cause of NASAH [1]. Third, many other vascular lesions may cause NASAH [5,9,24,26]. In this study, DSA and MRI performed in patients were not able to identify any possible causes described above, such as primitive venous drainage patterns, vascular malformations or tiny aneurysms that might be the cause.
NASAH is known to have a lower incidence than aneurysmal SAH but a better prognosis [12,13]. Factors related to favorable outcomes were young age, good neurological condition at hospitalization, no hydrocephalus, and a perimesencephalic pattern rather than aneurysmal pattern [4,13]. In the literature review, 90–100% of NASAH patients showed good recovery, and independent daily life was possible with no symptoms or only a few symptoms. Although less frequent than complications that occur after aneurysmal SAH, complications that occur after NASAH include rebleeding, delayed cerebral ischemia, and hydrocephalus. The incidence was 0.9%, 2.3%, and 9.3%, respectively [12]. As with previously reported results, most of the patients in this study also showed good clinical outcomes. There was no statistical significance due to the small number of patients, but there was one patient with a poor clinical outcome in the aneurysmal pattern. There was no patient who showed rebleeding or vasospasm, and there was one hydrocephalus, but it was not symptomatic and did not require treatment, so it is under regular follow-up.
The morphology of the basilar tip was classified into three types: symmetric cranial fusion, symmetric caudal fusion, and asymmetric fusion [3,17]. BA was formed after the fusion of the ventral longitudinal artery in front of the pontomesencephalic sulcus, and the extent of the fusion was determined by regression of the trigeminal artery at 5- to 9- mm (31–36 days) embryological stage [19]. When degeneration of the trigeminal artery occurs, if the flow of the internal carotid artery is more dominant than that of the vertebral artery, fusion of the ventral longitudinal artery does not proceed further and becomes a caudal fusion. In the opposite case, it becomes a cranial fusion [3]. The frequencies of the three fusion types are reported differently in different studies. In previous studies, the symmetric cranial fusion type of the basilar tip was the most common morphology. The caudal type in which the SCA branches from the PCA was reported to be 1–22% [18]. However, other studies reported that the frequency of caudal fusion type was 69.6–91%, of which the symmetric caudal fusion type was 26.1–56%, and the asymmetric fusion type was 23–43.5% [3,17]. In this study, 85.7% (n=18) of NASAH patients had a caudal fusion type, and only 14.3% (n=3) had a cranial fusion type. A total of 77.8% (n=7) of patients with the perimesencephalic pattern had a caudal fusion, and 91.7% (n=11) of patients with the aneurysmal pattern had a caudal fusion. We speculated in the case of the caudal fusion type, the length of the basilar artery is relatively shorter than cranial fusion type. Therefore, the perforators originating around the basilar tip are longer than those of the cranial type, and more of them are exposed to the subarachnoid space. This is thought to be more vulnerable to hemodynamic stress and contributed to NASAH incidence.
There are several limitations in this study. This study was conducted in a single-center, and the number of subjects was small. Because of the lack of an appropriate control group, no meaningful interpretation was suggested except that the basilar tip variant was more frequent in NASAH. Although this study speculated that NASAH is arterial bleeding according to the basilar tip variant, there is no consensus on whether NASAH is of arterial or venous origin. In the future, additional studies are needed to overcome these limitations and achieve appropriate clinical significance.
The basilar tip anatomy of the caudal fusion type tends to occur more frequent in both patients with the perimesencephalic pattern and patients with the aneurysmal pattern of NASAH. In the case of the caudal fusion type, compared to the cranial fusion type, more perforators around the basilar tip are exposed to the subarachnoid space, which is thought to be more vulnerable to hemodynamic stress. Further studies are needed to elucidate the clinical relationship between NASAH and the basilar tip variant.
REFERENCES
1. Alexander MS, Dias PS, Uttley D. Spontaneous subarachnoid hemorrhage and negative cerebral panangiography. Review of 140 cases. J Neurosurg. 1986; Apr. 64(4):537–42.
2. Beseoglu K, Pannes S, Steiger HJ, Hanggi D. Long-term outcome and quality of life after nonaneurysmal subarachnoid hemorrhage. Acta Neurochir (Wien). 2010; Mar. 152(3):409–16.

3. Campos C, Churojana A, Rodesch G, Alvarez H, Lasjaunias P. Basilar tip aneurysms and basilar tip anatomy. Interv Neuroradiol. 1998; Jun. 4(2):121–5.

4. Canovas D, Gil A, Jato M, de Miquel M, Rubio F. Clinical outcome of spontaneous non-aneurysmal subarachnoid hemorrhage in 108 patients. Eur J Neurol. 2012; Mar. 19(3):457–61.

5. Cioffi F, Pasqualin A, Cavazzani P, Da Pian R. Subarachnoid haemorrhage of unknown origin: clinical and tomographical aspects. Acta Neurochir (Wien). 1989; 97(1-2):31–9.

6. Claassen J, Bernardini GL, Kreiter K, Bates J, Du YE, Copeland D, et al. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: the Fisher scale revisited. Stroke. 2001; Sep. 32(9):2012–20.

7. Etminan N, Chang HS, Hackenberg K, de Rooij NK, Vergouwen MDI, Rinkel GJE, et al. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and meta-analysis. JAMA Neurol. 2019; May. 76(5):588–97.

8. Flaherty ML, Haverbusch M, Kissela B, Kleindorfer D, Schneider A, Sekar P, et al. Perimesencephalic subarachnoid hemorrhage: incidence, risk factors, and outcome. J Stroke Cerebrovasc Dis. 2005; Nov-Dec. 14(6):267–71.

9. Hashimoto H, Iida J, Shin Y, Hironaka Y, Sakaki T. Spinal dural arteriovenous fistula with perimesencephalic subarachnoid haemorrhage. J Clin Neurosci. 2000; Jan. 7(1):64–6.

10. Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968; Jan. 28(1):14–20.

11. Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975; Mar. 1(7905):480–4.
12. Kapadia A, Schweizer TA, Spears J, Cusimano M, Macdonald RL. Nonaneurysmal perimesencephalic subarachnoid hemorrhage: diagnosis, pathophysiology, clinical characteristics, and long-term outcome. World Neurosurg. 2014; Dec. 82(6):1131–43.

13. Konczalla J, Platz J, Schuss P, Vatter H, Seifert V, Guresir E. Non-aneurysmal non-traumatic subarachnoid hemorrhage: patient characteristics, clinical outcome and prognostic factors based on a single-center experience in 125 patients. BMC Neurol. 2014; Jul. 14:140.

14. Kurosu A, Suzukawa K, Amo M, Horinaka N, Arai H. Perimesencephalic non-aneurysmal subarachnoid hemorrhage caused by cavernous sinus thrombosis: case report. Neurol Med Chir (Tokyo). 2007; Jun. 47(6):258–60.
15. Linn FH, Rinkel GJ, Algra A, van Gijn J. Incidence of subarachnoid hemorrhage: role of region, year, and rate of computed tomography: a meta-analysis. Stroke. 1996; Apr. 27(4):625–9.
16. Mathews MS, Brown D, Brant-Zawadzki M. Perimesencephalic nonaneurysmal hemorrhage associated with vein of Galen stenosis. Neurology. 2008; Jun. 70(24 Pt 2):2410–1.

17. Matsukawa H, Kamiyama H, Noda K, Ota N, Takahashi O, Shonai T, et al. Embryological basilar apex disposition as a risk factor of basilar apex aneurysm. J Clin Neurosci. 2018; Dec. 58:79–82.

18. Meguro T, Taniguchi M, Hamauchi S, Onishi M, Fukuhara T, Miyoshi Y, et al. A case of ruptured cerebral aneurysm with asymmetric fusion of basilar apex. Journal of Neuroendovascular Therapy. 2021; 15(2):120–3.

19. Okahara M, Kiyosue H, Mori H, Tanoue S, Sainou M, Nagatomi H. Anatomic variations of the cerebral arteries and their embryology: a pictorial review. Eur Radiol. 2002; Oct. 12(10):2548–61.

20. Park SQ, Kwon OK, Kim SH, Oh CW, Han MH. Premesencephalic subarachnoid hemorrhage: rupture of tiny aneurysms of the basilar artery perforator. Acta Neurochir (Wien). 2009; Dec. 151(12):1639–46.

21. Quinn TJ, Dawson J, Walters M. Dr John Rankin; his life, legacy and the 50th anniversary of the Rankin Stroke scale. Scott Med J. 2008; Feb. 53(1):44–7.

22. Rinkel GJ, Wijdicks EF, Vermeulen M, Hasan D, Brouwers PJ, van Gijn J. The clinical course of perimesencephalic nonaneurysmal subarachnoid hemorrhage. Ann Neurol. 1991; May. 29(5):463–8.

23. Sangra MS, Teasdale E, Siddiqui MA, Lindsay KW. Perimesencephalic nonaneurysmal subarachnoid hemorrhage caused by jugular venous occlusion: case report. Neurosurgery. 2008; Dec. 63(6):E1202–3; discussion E1203.
24. Schievink WI, Wijdicks EF. Origin of pretruncal nonaneurysmal subarachnoid hemorrhage: ruptured vein, perforating artery, or intramural hematoma? Mayo Clin Proc. 2000; Nov. 75(11):1169–73.

25. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974; Jul. 2(7872):81–4.
26. Thines L, Khalil C, Fichten A, Lejeune JP. Spinal arachnoid cyst related to a nonaneurysmal perimesencephalic subarachnoid hemorrhage: case report. Neurosurgery. 2005; Oct. 57(4):E817.

Fig. 1.
Perimesencephalic patterns, aneurysmal patterns and basilar tip anatomy on a 3-dimensional reconstruction image of digital subtraction angiography. (A) Brain CT shows perimesencephalic pattern. Hemorrhage exists around the basal cistern with little extension to other cisterns. (B) Brain CT shows aneurysmal pattern. Hemorrhage is distributed beyond the basal cistern. (C) Bilateral superior cerebellar arteries (SCAs) originate from the basilar artery (BA) (white arrows), which shows a cranial fusion type. (D) Bilateral SCAs originate from the posterior cerebral artery (PCA) (white arrows), which shows a symmetric caudal fusion type. (E) The right SCA originates from the PCA (white arrowhead), and the left SCA originates from the BA (white arrow). This shows the asymmetric fusion type. CT, computed tomography
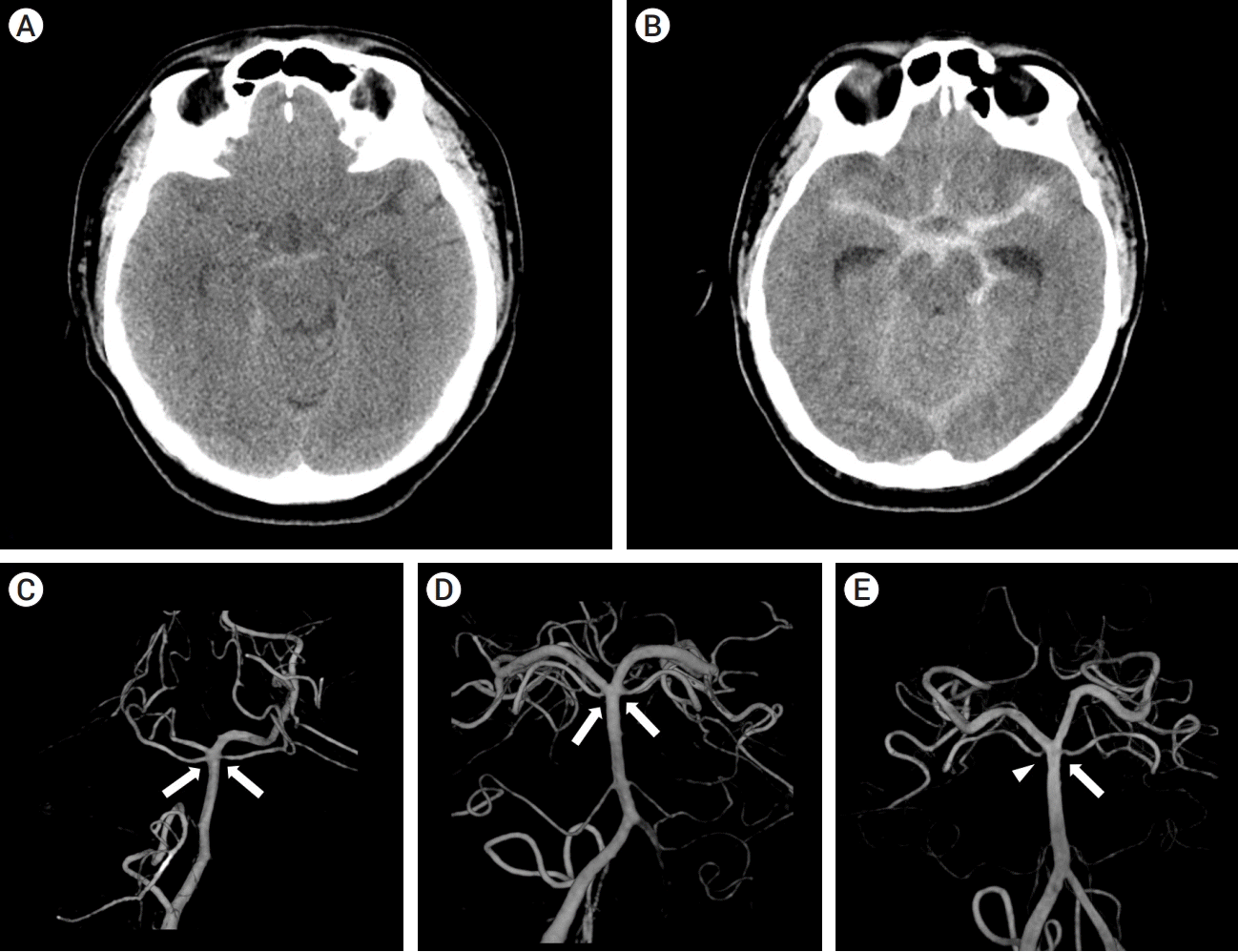
Fig. 2.
(A) Paired initial modified Rankin Scale (mRS) and the last follow-up mRS. Most of the patients with good clinical outcomes improved the last follow-up mRS compared to the initial mRS. A patient who showed poor clinical outcome had initial mRS 4 and the last follow-up mRS 4. (B) Paired initial Glasgow Coma Scale (GCS) and the last follow-up GCS. In all patients, the last follow-up GCS was equal to or better than the initial GCS.
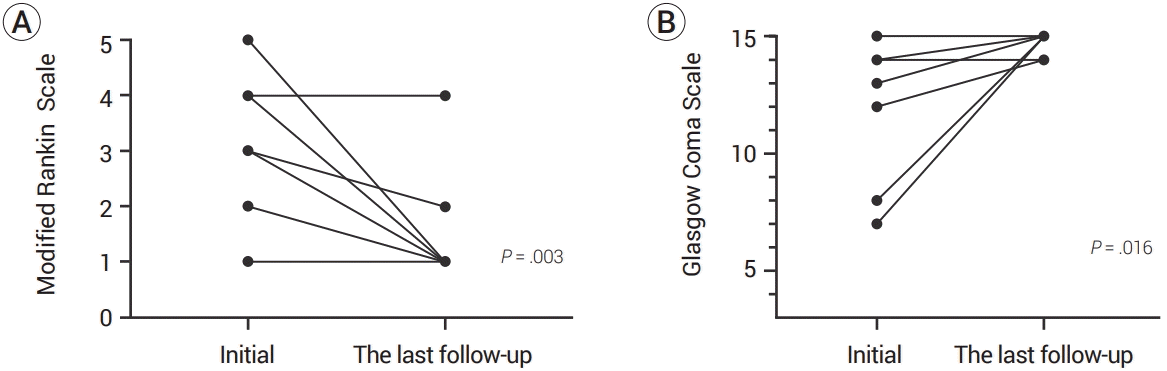
Table 1.
Demographic and clinical features of non-aneurysmal spontaneous subarachnoid hemorrhage
Table 2.
Clinical outcomes of non-aneurysmal spontaneous subarachnoid hemorrhage
Table 3.
Basilar tip anatomy and other angiographic findings of non-aneurysmal spontaneous subarachnoid hemorrhage




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download