Abstract
Remote cerebellar hemorrhage (RCH) is a rare complication of neurosurgical procedures and is characterized by a typical bleeding pattern defined as the “zebra sign.” Only few cases of RCH have been reported in the English literature, and its pathophysiology remains unclear. In this report, we present the cases of three patients with RCH after three different procedures: burr-hole trephination and chronic subdural hematoma evacuation of bilateral cerebral convexity with subsequent subdural drain insertion, lumbar drainage for cerebrospinal fluid divergence for thoracic endovascular aortic repair, and combined bypass surgery for moyamoya disease.
Remote cerebellar hemorrhage (RCH) is a rare postoperative complication that occurs remotely from the site of a neurosurgical procedure. Its incidence is approximately 0.3% following supra-tentorial surgery and is much rarer following spinal surgery [5,7,10]. RCH is usually characterized by a “zebra sign” in approximately 65% of patients and is associated with a good prognosis; otherwise, pure intracerebral hemorrhage indicates a poor prognosis [9].
From 2017 to 2020, we managed three cases of RCH that resulted in poor prognosis after 1) burr-hole trephination and subdural hemorrhage (SDH) evacuation, 2) lumbar drainage for cerebrospinal fluid (CSF) divergence for thoracic endovascular aortic repair, and 3) combined bypass surgery for moyamoya disease (MMD).
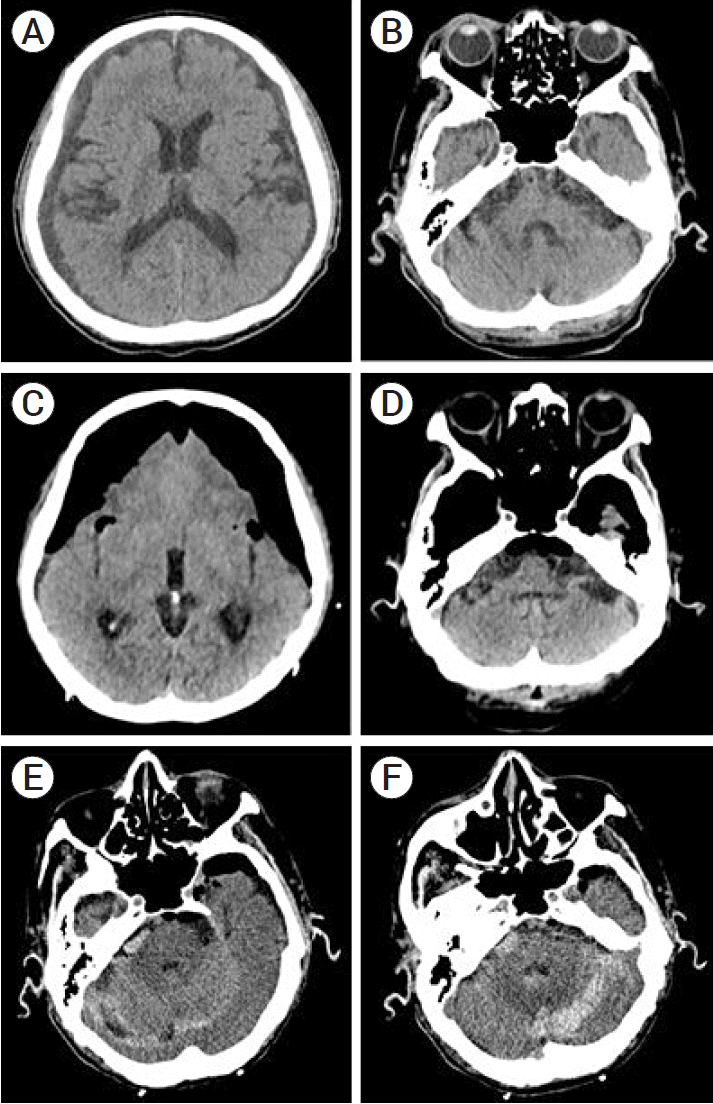
An 87-year-old man presented with the complaint of weakness in both legs since 2 days before presentation. His medical history included head trauma that had occurred a week ago, type 2 diabetes mellitus, and Parkinson’s disease. Brain computed tomography (CT) showed bilateral chronic SDH. Burr-hole trephination and SDH evacuation were performed, and a subdural drain was inserted bilaterally (Fig. 1A-D). To reduce the risk of pneumocephalus, rapid catheterization and saline infusion were performed.
However, on the postoperative CT, a large amount of pneumocephalus with the Mt. Fuji sign was observed. Oxygen administration using a facial oxygen mask was performed to facilitate oxygen absorption.
Nevertheless, his mental status gradually declined from glasgow coma scale (GCS) 13 (E3M6V4) to 9 (E2M5V1). To relieve intracranial pressure (ICP) resulting from increased air volume, air aspiration was performed through a three-way valve of the subdural drain. After the aspiration of a total of 80 cc of air, he spontaneously opened his eyes and recovered with GCS E4M6V3. On the follow-up CT scan, tension pneumocephalus was relieved, but unexpected bilateral RCH appeared (Fig. 1E, 1F). The bleeding did not increase during observation, but the patient developed symptoms associated with cerebellar hemorrhage and did not show improvement. Cerebellar mutism and swallowing difficulty were noted. Moreover, pneumonia and sepsis occurred. Over 4 weeks, the patient was stabilized and transferred to the rehabilitation center. However, recurrent pneumonia occurred after discharge, and the patient eventually expired.
A 70-year-old man was admitted for an elective operation for recently increased chronic type B aortic dissection. He had hypertension, which was effectively controlled using a calcium channel blocker. He also had stage III chronic kidney disease. Brain magnetic resonance imaging (MRI) was performed as a preoperative workup, which showed no significant abnormal findings such as vascular malformation. He had no focal neurologic deficit.
Thoracic endovascular aortic repair (TEVAR) was performed. Lumbar drainage catheter was inserted after general anesthesia to prevent spinal cord ischemia after TEVAR. Data about the amount of intraoperative CSF drainage were not obtained. The operative time was 2 h, and the patient woke up to GCS E3M6Ve and could follow simple commands postoperatively.
On postoperative day 1, the lumbar drainage catheter was removed after confirming that the strength of both legs was normal. A total of 210 cc of CSF was drained for 20 h postoperatively.
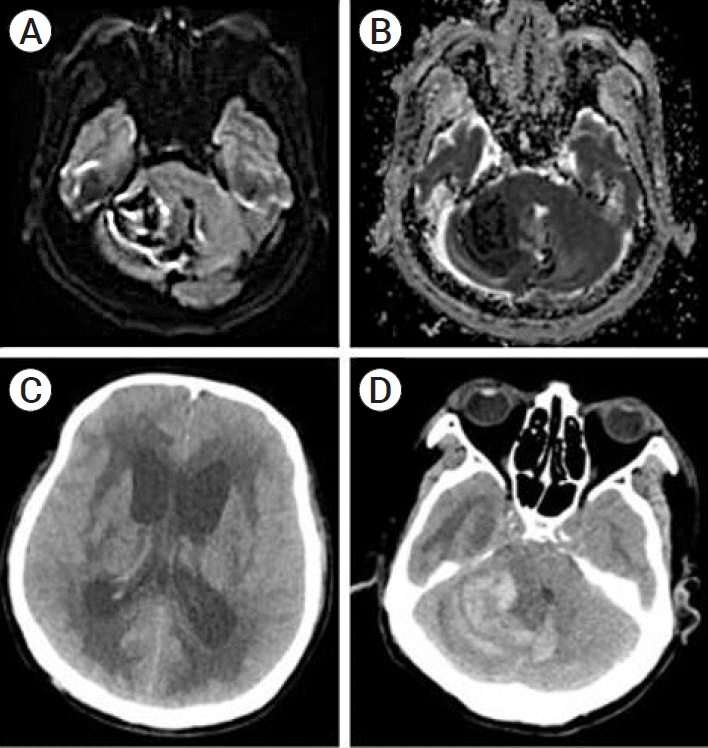
From the night of postoperative day 1, his level of consciousness gradually deteriorated to E1M4V. Brain MRI was performed, which revealed a large amount of bilateral cerebellar hemorrhage compressing the brainstem (Fig. 2A, 2B). When the patient was re-evaluated after the MRI, no brainstem reflexes were observed. Osmotic therapy was initiated, but the patient did not show any clinical improvement. The patient’s family decided not to pursue surgical decompression due to his poor prognosis. Follow-up brain CT showed aggravated hydrocephalus without RCH changes (Fig. 2C, 2D). The patient died 17 days after the onset of RCH.
A 55-year-old woman who presented with transient bilateral limb weakness and motor dysphasia was diagnosed with MMD and admitted for right combined bypass surgery. She also had hyperlipidemia and no focal neurologic deficit. Brain MRI and MR angiography showed typical characteristics of hemodynamically unstable MMD with acute Rt. border-zone infarction.
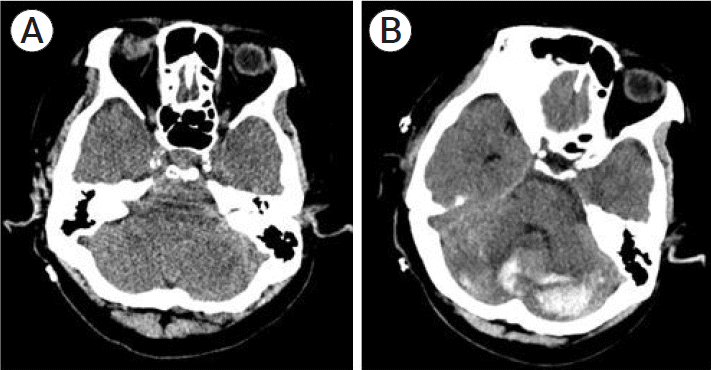
The patient underwent right combined bypass surgery as scheduled. The surgery was performed in the supine position, and the recipient artery was the angular branch of the right middle cerebral artery. The total operative time was 5 h, and the ischemic time was 59 min. No significant event occurred during surgical procedures. The patient was transferred to the intensive care unit following postoperative CT. Although no specific findings were observed on CT, the patient did not recover from anesthesia for 3 h and her level of consciousness was E1M4V at best. A total of 165 cc of fluid was drained through the sub-galea drainage for 3 h postoperatively. A 2nd CT was performed to check for any delayed adverse events and RCH was confirmed (Fig. 3).
She gradually recovered from anesthesia to E4M6Ve during close observation, and RCH did not increase on the follow-up CT. However, on the morning of postoperative day 3, stupor (E2M5V1) and grade II left side weakness were noted. Brain MRI revealed an acute infarction of the right frontal lobe. Vigorous hydration and induced hypertension were initiated. The patient gradually recovered her consciousness level to E4M6V2, but left side weakness persisted. She was transferred to the rehabilitation center after 1 month. Unfortunately, no significant change in the patient’s status was observed until the 3-year postoperative follow-up.
Many authors agree that RCH originates from the veins and occurs due to excessive CSF drainage during a neurosurgical procedure, which induces downward displacement of the cerebellum and stretching of the cerebellar veins [3,6,7]. However, its actual pathophysiology remains unclear.
Schievink et al. reviewed 262 patients with spontaneous intracranial hypotension and demonstrated that chronic cerebellar hemorrhage was associated with CSF leakage (p<0.0001) [8]. It indirectly demonstrates an association between CSF leakage and RCH statistically. Similarly, two cases (Cases 2 and 3) presented in this case report were also associated with perioperative CSF drainage, and the onset of RCH was within 48 h after the neurosurgical procedure. However, no cutoff value of the CSF drainage volume has been established for RCH occurrence.
Moreover, there are few reports describing the occurrence of RCH even after minimal CSF drainage. Therefore, the amount of CSF alone is not sufficient to explain the pathophysiology of RCH. Bokhari et al. presented the case of an infant with RCH after a ventriculoperitoneal shunt operation, although only 5 cc of CSF drainage was obtained during the procedure [2]. Similarly, Hara et al. reported the case of a patient with RCH after supratentorial glioma removal without notable CSF leakage [4]. They suggested that RCH is more significantly associated with ICP changes. A direct force that leads to venous rupture might be the total transmural pressure gradient and shear stress induced by rapid ICP changes. Perhaps this pathophysiology is applicable to Case 1 reported herein. A sudden change in ICP would have occurred due to the sudden inhalation of air in a situation in which tension pneumocephalus existed.
Taking it one step further, a sharp decrease in ICP can lead to the congestion of the cerebral vein structures, thereby causing turbulence in the engorged vein and activating the extrinsic coagulation pathway. Andrews et al. reported a case in which cerebellar hemorrhage and infarction occurred simultaneously after a massive CSF leak due to a lumbar dural defect [1]. Friedman et al. revealed the presence of venous infarction on their histopathological review of a case of RCH.
Nevertheless, rapid changes in CSF drain and ICP do not cause RCH in all patients. To elucidate the pathophysiology of RCH, it will be necessary to discuss the patient’s risk factors. However, this remains challenging owing to the rarity of this complication.
Approximately 75% of patients with RCH do not require surgical management and can be sufficiently managed with conservative management [9]. However, RCH can be fatal if the brainstem is compressed by cerebellar hemorrhage, as in our second case.
RCH should also be considered when reviewing CT for unexplained neurological deterioration or delayed recovery of consciousness after surgery. If RCH is confirmed, appropriate ICP control is required. Further, if brain stem compression is suspected, surgical decompression should be considered.
REFERENCES
1. Andrews RT, Koci TM. Cerebellar herniation and infarction as a complication of an occult postoperative lumbar dural defect. AJNR Am J Neuroradiol. 1995; Jun-Jul. 16(6):1312–5.
2. Bokhari R, Baeesa S. Remote cerebellar hemorrhage due to ventriculoperitoneal shunt in an infant: A case report. J Med Case Rep. 2012; Jul. 6:222.

4. Hara T, Matsuda M, Watanabe S, Nakai K, Yamamoto T, Matsumura A. Remote cerebellar hemorrhage after removal of a supratentorial glioma without perioperative CSF loss: A case report. Case Rep Surg. 2013; 2013:305039.

5. Honegger J, Zentner J, Spreer J, Carmona H, Schulze-Bonhage A. Cerebellar hemorrhage arising postoperatively as a complication of supratentorial surgery: A retrospective study. J Neurosurg. 2002; Feb. 96(2):248–54.
6. Kelley GR, Johnson PL. Sinking brain syndrome: Craniotomy can precipitate brainstem herniation in CSF hypovolemia. Neurology. 2004; Jan. 62(1):157.

7. Park JS, Hwang JH, Park J, Hamm IS, Park YM. Remote cerebellar hemorrhage complicated after supratentorial surgery: Retrospective study with review of articles. J Korean Neurosurg Soc. 2009; Aug. 46(2):136–43.

8. Schievink WI, Maya MM, Nuno M. Chronic cerebellar hemorrhage in spontaneous intracranial hypotension: Association with ventral spinal cerebrospinal fluid leaks: Clinical article. J Neurosurg Spine. 2011; Oct. 15(4):433–40.
9. Sturiale CL, Rossetto M, Ermani M, Volpin F, Baro V, Milanese L, et al. Remote cerebellar hemorrhage after supratentorial procedures (part 1): A systematic review. Neurosurg Rev. 2016; Oct. 39(4):565–73.

10. Toczek MT, Morrell MJ, Silverberg GA, Lowe GM. Cerebellar hemorrhage complicating temporal lobectomy. Report of four cases. J Neurosurg. 1996; Oct. 85(4):718–22.
Fig. 1.
(A, B) Preoperative brain CT demonstrating bilateral subdural hemorrhage. (C, D) Immediate postoperative brain CT showing pneumocephalus with the Mt. Fuji sign. No lesions were observed in the cerebellum. (E, F) Follow-up brain CT showing newly emerged cerebellar hemorrhage. CT, computed tomography




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download