Abstract
We report on changes in the ascending reticular activating system (ARAS) concurrent with the recovery of impaired consciousness following rehabilitation and cranioplasty in a patient with traumatic brain injury (TBI), which were demonstrated on diffusion tensor tractography (DTT). A 34-year-old male patient was diagnosed with a traumatic intracerebral hemorrhage after falling from a height of approximately 7 m and underwent a right frontoparietotemporal decompressive craniectomy and hematoma removal. At 5 months after onset, when starting rehabilitation, the patient showed impaired consciousness, with a Glasgow Coma Scale (GCS) score of 4. Comprehensive rehabilitative therapy was provided until 14 months after onset, and his GCS score improved to 8. Cranioplasty was performed using auto-bone at 14 months after onset. One month after cranioplasty, his GCS score improved to 12. On the 15-month DTT, the deviated lower dorsal ARAS was restored on both sides, and the right side had become thicker. The right lower ventral ARAS was reconstructed, and increased neural connectivity of the upper ARAS was detected in both the prefrontal cortices. Thus, changes in the ARAS were demonstrated in a patient with TBI during recovery of consciousness following rehabilitation and cranioplasty.
The ascending reticular activating system (ARAS) is considered an important neural structure for the control of consciousness [1,2]. In the field of neuroscience, clarification of the neural structures of the ARAS involved in the recovery of consciousness has been an important topic with regard to neurorehabilitation in patients with disorders of consciousness. Diffusion tensor tractography (DTT), which is reconstructed from diffusion tensor imaging (DTI), has enabled the three-dimensional reconstruction of the ARAS, and several DTT studies have reported on changes in the ARAS in patients who showed recovery of impaired consciousness following rehabilitation [3-9]. In contrast, only one study reported the positive effect of cranioplasty on impaired consciousness without evidence of change in the ARAS [10].
In this study, we report on changes in the ARAS concurrent with the recovery of impaired consciousness following rehabilitation and cranioplasty in a patient with traumatic brain injury (TBI), which were demonstrated on DTT.
Ethical statements: This study was approved by the Institutional Review Board (IRB) of Yeungnam University Hospital (IRB No: YUMC-2021-03-014), and informed consent was obtained from the patient.
A 34-year-old, right-handed male patient was diagnosed with a traumatic intracerebral hemorrhage after falling from a height of approximately 7 meters and underwent a right frontoparietotemporal decompressive craniectomy and hematoma removal at the neurosurgery department of a local hospital. He was admitted to the rehabilitation department of a university hospital at 5 months after onset. Impaired consciousness was observed in the patient, with a Glasgow Coma Scale (GCS) score of 4 (eye opening, 1; best verbal response, 1; and best motor response, 2) [11]. Comprehensive rehabilitative therapy, including neurotropic drugs (levodopa, bromocriptine, baclofen, zolpidem, and amantadine), occupational therapy, and physical therapy, was provided [12]. After 9 months of rehabilitation (14 months after onset) at the university hospital and a local rehabilitation hospital, his GCS score improved to 8 (eye opening, 4; best verbal response, 1; and best motor response, 3) [11]. Cranioplasty was performed using auto-bone at 14 months after onset. One month after cranioplasty (15 months after onset), his GCS score improved to 12 (eye opening, 4; best verbal response, 2; and best motor response, 6), and he was able to open his eyes upon verbal command.
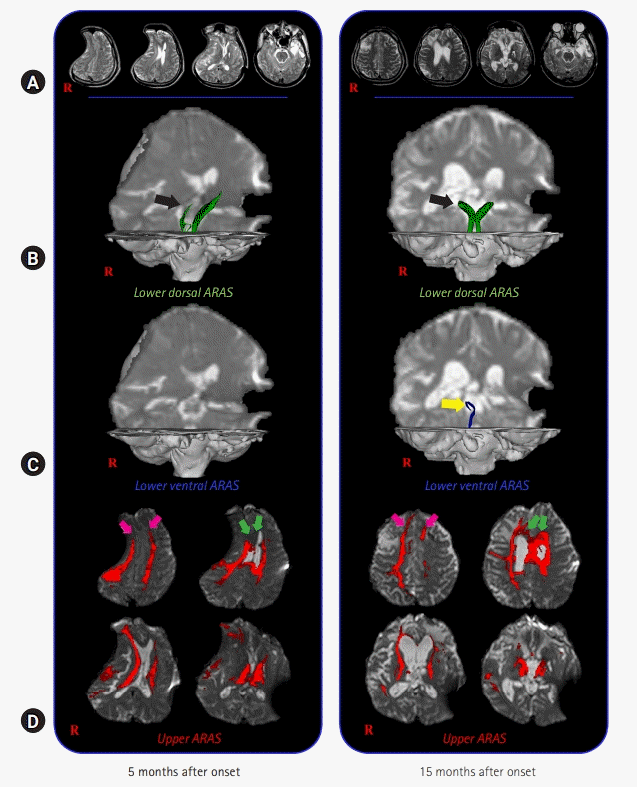
DTI data were acquired twice (5 months and 15 months after onset) using a 6-channel head coil on a 1.5 T Philips Gyroscan Intera (Philips, Best, Netherlands) with single-shot echo-planar imaging (Fig. 1A). Sixty-five contiguous slices (reconstruction matrix, 192×192 matrix; acquisition matrix, 96×96; echo time, 76 ms; field of view, 240×240 mm2; repetition time, 10,726 ms; number of excitations, 1; slice gap, 0 mm; thickness, 2.5 mm; b, 1,000 sec/mm2) were acquired for each of the 32 noncollinear diffusion-sensitizing gradients. The Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) software library was used to analyse DTI data. FMRIB Diffusion Software with routine options (curvature thresholds of 0.2, 5,000 streamline samples, and 0.5-mm step lengths) was used for fiber tracking. Three portions of the ARAS were analyzed by the selection of fibers passing through the following regions of interest (ROIs): the dorsal lower ARAS–the seed ROI was located on the pontine reticular formation (RF), and target ROI was placed on the intralaminar thalamic nucleus (ILN) [13]; the ventral lower ARAS–the seed ROI was placed on the pontine RF and target ROI located on the hypothalamus [14]; and the upper ARAS–seed ROI placed on the neural connectivity of the ILN to the cerebral cortex was analyzed [15].
On the 5-month DTT, in the lower dorsal ARAS, the upper portions were deviated to the left side and thin on the right side (Fig. 1B). The lower ventral ARAS was not reconstructed on both sides, and decreased neural connectivity was detected in both prefrontal cortices and both basal forebrains of the upper ARAS (Fig. 1C, 1D), while on 15-month DTT, the deviated lower dorsal ARAS was restored on both sides and the thinned right side had become thicker (Fig. 1B). The right lower ventral ARAS was reconstructed, and increased neural connectivity was detected in both the anterior cingulums and prefrontal cortices of the upper ARAS (Fig. 1C, 1D).
In this study, using DTT, changes in the ARAS were observed in a patient with TBI who showed recovery from a vegetative state to a minimally conscious state after comprehensive rehabilitation and cranioplasty; in detail, GCS, 4 (5 months after onset, first DTT); GCS, 8 (14 months after onset, before cranioplasty); and GCS, 12 (15 months after onset, after 1 month of cranioplasty, second DTT). In particular, 1 month before and after cranioplasty, he showed a 4-point improvement in the GCS score. The changes in the ARAS observed on DTT during the 10-month period from 5 months to 15 months after onset are as follows: (1) lower dorsal ARAS, normalization of bent configuration and thickening on the right side; (2) lower ventral ARAS, appearance on the right side; and (3) upper ARAS, the neural connectivity to both the anterior cingulums and prefrontal cortex was increased. The patient showed improved awareness (GCS, best motor response: 2 [5 months after onset, first DTT] to 3 [14 months after onset, before cranioplasty] to 6 [15 months after onset, after 1-month cranioplasty, second DTT]), rather than alertness (GCS, eye opening: 4 [5 months after onset, first DTT] to 4 [14 months after onset, before cranioplasty] to 4 [15 months after onset, 1 month after cranioplasty, second DTT]). Therefore, we believe that the increased neural connectivity to both prefrontal cortices and cingulums in the upper ARAS concurrent with the change in the lower ARAS was responsible for the improvement of consciousness in this patient. In addition, our results appeared to correspond with the results of previous studies reporting increased connectivity to the anterior cingulum and prefrontal cortex, which are important areas of awareness in the brain [3-9,16].
In conclusion, changes in the ARAS were observed in a patient with TBI who showed recovery of awareness following comprehensive management, including rehabilitation and cranioplasty. The increased neural connectivity of the prefrontal cortex and cingulum contributed to the recovery of awareness in this patient. We believe that our study has important implications for the management of patients with disorders of consciousness. However, several limitations of DTI should be considered. First, the fiber tracking technique is operator-dependent. Second, DTI may underestimate fiber tracts. DTI is a powerful anatomic imaging tool that can demonstrate gross fiber architecture, but not functional or synaptic connections. Third, regions of fiber complexity and crossing can prevent full reflection of the underlying fiber architecture by DTI [17,18].
Notes
References
1. Paus T. Functional anatomy of arousal and attention systems in the human brain. Prog Brain Res. 2000; 126:65–77.

3. Jang SH, Kim SH, Lim HW, Yeo SS. Recovery of injured lower portion of the ascending reticular activating system in a patient with traumatic brain injury. Am J Phys Med Rehabil. 2015; 94:250–3.

4. Jang SH, Chang CH, Jung YJ, Seo YS. Change of ascending reticular activating system with recovery from vegetative state to minimally conscious state in a stroke patient. Medicine (Baltimore). 2016; 95:e5234.

5. Jang SH, Hyun YJ, Lee HD. Recovery of consciousness and an injured ascending reticular activating system in a patient who survived cardiac arrest: a case report. Medicine (Baltimore). 2016; 95:e4041.
6. Jang SH, Kwon YH. Effect of repetitive transcranial magnetic stimulation on the ascending reticular activating system in a patient with disorder of consciousness: a case report. BMC Neurol. 2020; 20:37.

7. Jang SH, Lee HD. Recovery of an injured ascending reticular activating system with recovery from a minimally conscious state to normal consciousness in a stroke patient: a diffusion tensor tractography study. Neural Regen Res. 2020; 15:1767–8.

8. Jang SH, Seo YS, Lee SJ. Increased thalamocortical connectivity to the medial prefrontal cortex with recovery of impaired consciousness in a stroke patient: a case report. Medicine (Baltimore). 2020; 99:e19937.
9. Jang SH, Kim SH, Seo JP. Long-term recovery from a minimally responsive state with recovery of an injured ascending reticular activating system: a case report. Medicine (Baltimore). 2021; 100:e23933.
10. Stelling H, Graham L, Mitchell P. Does cranioplasty following decompressive craniectomy improve consciousness? Br J Neurosurg. 2011; 25:407–9.

11. Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974; 2:81–4.
12. Schiff ND. Recovery of consciousness after brain injury: a mesocircuit hypothesis. Trends Neurosci. 2010; 33:1–9.

13. Yeo SS, Chang PH, Jang SH. The ascending reticular activating system from pontine reticular formation to the thalamus in the human brain. Front Hum Neurosci. 2013; 7:416.

14. Jang SH, Kwon HG. The ascending reticular activating system from pontine reticular formation to the hypothalamus in the human brain: a diffusion tensor imaging study. Neurosci Lett. 2015; 590:58–61.

15. Jang SH, Lim HW, Yeo SS. The neural connectivity of the intralaminar thalamic nuclei in the human brain: a diffusion tensor tractography study. Neurosci Lett. 2014; 579:140–4.

16. Laureys S, Faymonville ME, Luxen A, Lamy M, Franck G, Maquet P. Restoration of thalamocortical connectivity after recovery from persistent vegetative state. Lancet. 2000; 355:1790–1.

Fig. 1.
(A) Brain magnetic resonance images taken at 5 months and 15 months after onset show leukomalactic lesions in the right parietal and occipital lobes, both frontal and temporal lobes, and left basal ganglia. (B–D) Results of diffusion tensor tractography (DTT) for the ascending reticular activating system (ARAS) of the patient. On 5-month DTT, in the lower dorsal ARAS, the upper portions are deviated to the left side and thin on the right side (black arrow). The lower ventral ARAS is not reconstructed on both sides, and decreased neural connectivity of the upper ARAS is detected in both prefrontal cortices and both basal forebrains. By contrast, on 15-month DTT, the deviated lower dorsal ARAS is restored on both sides and the thinned right side has become thicker (black arrow). The right lower ventral ARAS (yellow arrow) is reconstructed and increased neural connectivity of the upper ARAS is detected in both prefrontal cortices (pink arrows) and anterior cingulums (green arrows).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download