Abstract
Background
Epidural analgesia is commonly used for pain control during lung cancer surgery. However, the clinical trends in epidural analgesia, associated factors, and their association with clinical outcomes remain controversial. Therefore, we aimed to investigate the trends, associated factors, and their association with the clinical outcomes of epidural analgesia for lung cancer surgery.
Methods
The National Health Insurance Database was used as the data source in a nationwide cohort study. All adult patients who underwent lung cancer surgery between 2011 and 2018 were included.
Results
A total of 60,031 adult patients who underwent surgery for lung cancer were included. Of these, a total of 24,786 patients (41.3%) received epidural analgesia with a mean value of 1.5 days (standard deviation: 2.0 days). Male sex, increased Charlson comorbidity index (CCI), concurrent musculoskeletal disease, and a wider surgical extent were associated with higher odds of epidural analgesia for lung cancer surgery. Compared to open thoracotomy, video-assisted thoracoscopic surgery (VATS) was associated with lower odds of epidural analgesia for lung cancer surgery. Moreover, epidural analgesia was not associated with 30-day mortality, fatal respiratory events, or one-year mortality after lung cancer surgery.
Conclusions
From 2011 to 2018, 41.3% of patients with lung cancer in South Korea received epidural analgesia for lung cancer surgery. Some factors (male sex, increased CCI, concurrent musculoskeletal disease, wider surgical extent, and VATS) were associated with the use of epidural analgesia in lung cancer surgery. However, epidural analgesia was not associated with clinical outcomes after lung cancer surgery.
Lung cancer is the leading cause of cancer-related mortality worldwide [1]. According to global cancer statistics from 185 countries in 2020 [2], 2,206,771 individuals were newly diagnosed with lung cancer, and 1,796,144 patients died due to lung cancer in 2020. As the global incidence, death, and economic burden of lung cancer have increased [3], effective management of lung cancer remains a significant public health issue.
For the treatment of lung cancer, surgical procedures are first considered with curative intent [4]. Patients who undergo lung cancer surgery are known to experience severe postoperative pain that might affect their quality of life [5,6]. Epidural analgesia has been widely used for clinical benefits, such as effective postoperative pain control during thoracotomy and attenuation of the inflammatory response during lung cancer surgery [7,8]. Thus, epidural analgesia is considered an optimal technique for effective pain control in lung cancer surgery, especially for open thoracotomy cases [9]. However, video-assisted thoracoscopic surgery (VATS), which is a minimally invasive surgical technique, has emerged as the standard surgical procedure for lung cancer surgery [10], and there is no clear gold standard for regional analgesia in lung cancer surgery [11]. For example, intravenous (IV) analgesia, thoracic paravertebral block, and intercostal nerve block have been used for postoperative pain control to replace epidural analgesia in lung cancer surgery [11]. Thus, the trend in the application of epidural analgesia has changed over time. However, no study has been conducted on the epidemiology of epidural analgesia for lung cancer surgery using a nationwide registration database.
Therefore, the present study aimed to investigate trends, associated factors, and their association with the clinical outcomes of epidural analgesia for lung cancer surgery using the South Korean national registration database.
The study protocol was approved by the Institutional Review Board (IRB) (IRB approval number: X-2008-630-902). The National Health Insurance Scheme (NHIS) approved the data sharing protocol for this study (NHIS approval number: NHIS-2021-1-041). The requirement for informed consent was waived by the IRB because the data were analyzed retrospectively in an anonymous form after masking the individual and sensitive information of the study population.
The NHIS database was used as the national registration database. The NHIS database contains all disease diagnoses and prescription information regarding the procedures and/or drugs in South Korea. The government supports financial expenses for treatment or medical examinations after registration of disease diagnoses and prescriptions. Moreover, the NHIS database contains demographic and socio-economic information of the South Korean population. The 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) codes is used to register any disease in the NHIS database.
This study included all adult patients (≥ 18 years old) who were diagnosed with lung cancer (C34 of ICD-10 code) and underwent lung cancer surgery between January 1, 2011, and December 31, 2018, in South Korea. In South Korea, the government covers almost all expenses (approximately 95%) involved in the treatment and examination of lung cancer after the registration of ICD-10 codes (C34); thus, all patients with lung cancer who underwent lung cancer surgery were included in the NHIS database.
As the main independent variable, information on the administration of epidural analgesia during lung cancer surgery for pain control was collected. The prescription code for epidural patient-controlled analgesia (PCA) was used to extract data. Patients who received epidural PCA after lung cancer surgery were considered the epidural analgesia group, and the other patients were considered the control group.
First, the proportion of patients who received epidural analgesia from 2011 to 2018 after lung cancer surgery was examined. Second, the factors associated with epidural analgesia in patients who underwent lung cancer surgery were examined. Third, the association of epidural analgesia with 30-day mortality, development of fatal respiratory events, and one-year mortality after lung cancer surgery was investigated. A fatal respiratory event was defined as a diagnosis of acute respiratory distress syndrome (ARDS) (J80 of ICD-10 code) or respiratory failure (J96 of ICD-10 code) during hospitalization following lung cancer surgery.
Age and sex were collected as demographic information. To reflect socioeconomic status, household income level, employment status, and residence locality at the time of lung cancer surgery were collected. The annual household income level in South Korea is registered by considering the individual’s annual income and property to determine their insurance premium. All patients were divided into four groups based on the quartile ratio of the household income level. Employment status did not include self-employed people, and all patients were divided into two groups according to their residence: urban areas (capital or other metropolitan cities) and rural areas (all other areas). For surgery-related information, the type of surgery, use of VATS or open thoracotomy, and a redo-case of surgery were collected for this study. Surgery type was divided into five groups: wedge resection, segmentectomy, lobectomy, bilobectomy, and pneumonectomy. If a patient underwent wedge resection in addition to segmentectomy, the patient was included in the segmentectomy group, whereas a patient who underwent segmentectomy in addition to lobectomy was included in the lobectomy group. As a high case volume was strongly associated with improved survival outcomes after lung cancer surgery [12], the annual number of lung cancer surgery cases in each hospital in South Korea was calculated. The patients were then divided into four groups using quartile ratios, based on the case load of the hospital in which the lung cancer surgery was performed (Q1: ≤ 74, Q2: 75–276, Q3: 277–921, and Q4: ≥ 922). In addition, all patients were divided into two groups according to the hospital in which the lung cancer surgery was performed: a tertiary general hospital group and a general hospital group. The total cost of hospitalization (United States Dollar [USD]) and length of hospital stay (days) were recorded. For comorbid stats related information, the Charlson comorbidity index (CCI) at the time of lung cancer surgery was calculated using the ICD-10 codes of individual diseases (Supplementary Table 1), which were registered within one year before the date of the lung cancer surgery. Concurrent musculoskeletal disease (M* of ICD-10 code) and preoperative chronic analgesic (opioid, paracetamol, non-steroidal anti-inflammatory drugs, gabapentin, or pregabalin) use (≥ 90 days) were collected as covariates. In addition, the underlying disability before lung cancer surgery was collected because all individuals with any disability should be registered in the NHIS database to receive various benefits from the social welfare system in South Korea. The disabilities were divided into six grades based on severity (grade 1, most severe; grade 6, mildest). Patients with grades 1, 2, or 3 constituted the severe disability group, while those with grades 4, 5, or 6 constituted the mild-to-moderate disability group.
The clinicopathological characteristics of all patients are presented as mean values with standard deviations (SDs) for continuous variables and numbers with percentages for categorical variables. For comparison of clinicopathological characteristics between the epidural analgesia and control groups, t-test and chi-square test were used. Next, we constructed a multivariable logistic regression model to examine the factors associated with epidural analgesia. All variables were included in the multivariable model for adjustment, and the Hosmer–Lemeshow test was used to confirm if the goodness of fit in the model was appropriate.
For secondary analyses, multivariable logistic models were constructed to investigate whether epidural analgesia was associated with 30-day mortality or development of fatal respiratory events after lung cancer surgery. We also fitted a multivariable Cox regression model to examine whether epidural analgesia was associated with the one-year mortality risk after lung cancer surgery. The results of logistic regression were presented as odds ratios (ORs) with 95% CIs, whereas those of Cox regression were presented as hazard ratios (HRs) with 95% CIs. There was no multicollinearity between the variables in the model with variance inflation factors < 2.0. All statistical analyses were performed using SAS (version 9.4; SAS Institute Inc., USA) and R software (version 3.6.2; R Foundation for Statistical Computing, Austria). Statistical significance was set at P < 0.05.
A total of 60,031 adult patients who were diagnosed with lung cancer and underwent lung cancer surgery between January 1, 2011, and December 31, 2018, were included in the analysis. Clinicopathological characteristics of the patients are shown in Table 1. The mean age of the patients was 65.6 years (SD: 9.9 years), and 61.3% (36,778/60,031) were men. The epidural analgesia group included 24,786 patients (41.3%), and they received epidural analgesia for a mean duration of 1.5 days (SD: 2.0 days).
Fig. 1 shows the trends in epidural analgesia use for lung cancer surgery in South Korea. In 2011, 45.2% (2,477/5,479) of patients received epidural analgesia, which gradually increased until 2016 (64.7%, 5,406/8,360). However, the proportion of epidural analgesics decreased by 20.8% (1,889/9,076) in 2017 and 12.0% (1,198/9,959) in 2018. Table 2 shows the results of the comparison of clinicopathological characteristics between the epidural analgesia and control groups. The epidural analgesia group had a higher proportion of lobectomy (17,055/24,786; 68.8% vs. 23,733/35,245; 67.3%), bilobectomy (972/24,786; 3.9% vs. 985/35,245; 2.8%), and pneumonectomy (928/24,786; 3.7% vs. 828/35,245; 2.3%) than the control group (P < 0.001).
Table 3 shows the results of the multivariate logistic regression model for the application of epidural analgesia. Male sex (OR: 1.17, 95% CI [1.12, 1.21], P < 0.001), increased CCI (OR: 1.06, 95% CI [1.06, 1.07], P < 0.001), and concurrent musculoskeletal disease (OR: 1.46, 95% CI [1.36, 1.57], P < 0.001) were associated with higher odds of epidural analgesia for lung cancer surgery. Compared to wedge resection, segmentectomy (OR: 1.13, 95% CI [1.04, 1.23], P = 0.003), lobectomy (OR: 1.21, 95% CI [1.16, 1.27], P < 0.001), bilobectomy (OR: 1.52, 95% CI [1.37, 1.69], P < 0.001), and pneumonectomy (OR: 1.68, 95% CI [1.50, 1.88], P < 0.001) were associated with higher odds of epidural analgesia for lung cancer surgery. Compared with open thoracotomy, VATS was associated with lower odds of epidural analgesia for lung cancer surgery (OR: 0.73, 95% CI [0.70, 0.77], P < 0.001).
Table 4 shows the results of the analyses regarding 30-day mortality, fatal respiratory events, and one-year mortality associated with epidural analgesia for lung cancer surgery. The epidural analgesia group showed no significant difference in the odds of 30-day mortality (OR: 1.07, 95% CI [0.87, 1.32], P = 0.522), fatal respiratory events (OR: 1.12, 95% CI [0.87, 1.45], P = 0.388), and one-year mortality risk (HR: 1.01, 95% CI [0.94, 1.07], P = 0.840). However, epidural analgesia might influence the management of acute and chronic pain control and patient satisfaction following lung cancer surgery. Unfortunately, these details are not available in the NHIS dataset.
According to the results of this population-based cohort study in South Korea, 41.3% patients received epidural analgesia for lung cancer surgery. Even though there has been a recent decreased rate of epidural analgesia with the increase of VATS, our study suggested that epidural analgesia might still be a good option in case of increased CCI, concurrent musculoskeletal disease, and wider surgical extent. Moreover, clinical outcomes were assessed, such as 30-day mortality, fatal respiratory events, and one-year mortality.
The most clinically relevant points in this study were the associated factors for the application of epidural analgesia because it reflects the favoring of epidural analgesia among anesthesiologists and surgeons for pain control of lung cancer surgery using real-world data. Although the risks and benefits of thoracic epidural analgesia for pain control have been documented [13], there was insufficient information on the cases in which epidural analgesia was frequently performed for pain control of lung cancer surgery. Moreover, we also showed that epidural analgesia might not affect important clinical outcomes after lung cancer surgery such as 30-day mortality, fatal respiratory events, and one-year mortality. This is the first study to describe the factors associated with the use of epidural analgesia for lung cancer surgery using real-world data based on a national registration database.
The efficacy of epidural analgesia in lung cancer surgery remains controversial [14]. As the thoracic epidural technique is a high-risk procedure that may cause dural puncture, epidural hematoma, or nerve damage [15], IV analgesia has been used for postoperative pain control in VATS as a less invasive technique. Kim et al. [16] reported that IV and epidural analgesia are equally effective for pain control in VATS lobectomy. Moreover, paravertebral block could be used instead of epidural analgesia for effective pain control in VATS [17]. In South Korea, the proportion of VATS among lung cancer surgeries has increased from 64.5% (3,535/5,479) in 2011 to 91.4% (9,106/9,959) in 2018 (Supplementary Fig. 1). The increasing trend of VATS in South Korea may have affected the decrease in epidural analgesia since 2016.
A wider surgical extent, such as pneumonectomy, bilobectomy, and lobectomy, might affect the preference for epidural analgesia in South Korea. In addition to postoperative pain control, epidural analgesia protects against pneumonia after lung cancer surgery [18]. Pneumonectomy for lung cancer is a high-risk procedure, and epidural analgesia lowers the risk of respiratory complications after pneumonectomy [19]. Thus, the anesthesiologists and thoracic surgeons used epidural analgesia for lobectomy, bilobectomy, and pneumonectomy more than for wedge resection and segmentectomy. Similarly, increased CCI with comorbid status might affect the clinician’s preference for epidural analgesia because patients with many underlying diseases have a high risk of pneumonia after lung cancer surgery [20]. Therefore, patients with lung cancer in addition to many other underlying diseases need epidural analgesia to prevent pneumonia after lung cancer surgery.
Importantly, epidural analgesia was not associated with clinical outcomes after lung cancer surgery, such as 30-day mortality, fatal respiratory events, or one-year mortality. This is a clinically important result because epidural analgesia is known to reduce pulmonary complications after lung cancer surgery [19,20]. A retrospective cohort study from a single medical center in Taiwan reported that thoracic epidural analgesia was not associated with better recurrence-free or overall survival in patients who underwent lung cancer surgery [21]. Moreover, in a recent randomized trial by Xu et al. [22] epidural analgesia did not improve overall, recurrence-free, or cancer-specific survival after major lung cancer surgery. In addition to the previous reports [21,22], the present epidemiological study conducted in South Korea also showed that the impact of epidural analgesia on clinical outcomes after lung cancer surgery was not significant.
This study had several limitations. First, preoperative lung function was not evaluated because of a lack of data in the NHIS database. For example, the forced expiratory volume per second was not used in this study. Second, lifestyle factors, such as alcohol consumption and smoking history, were not evaluated due to a lack of data sources. Third, the tumor stage of lung cancer was not provided in this study, which could have affected the use of epidural anesthesia and mortality after lung cancer surgery. Finally, generalizability might be limited because medical and insurance systems differ according to the country. For example, patients with lung cancer in South Korea had to pay approximately 5% of the total treatment expense, including epidural analgesia, due to financial support from the NHIS.
In conclusion, from 2011 to 2018, 41.3% of patients with lung cancer in South Korea received epidural analgesia for lung cancer surgery. Some factors (male sex, increased CCI, concurrent musculoskeletal disease, wider surgical extent, and VATS) were associated with the use of epidural analgesia in lung cancer surgery. Moreover, epidural analgesia was not associated with clinical outcomes (30-day mortality, fatal respiratory events, or one-year mortality) after lung cancer surgery. However, considering the limitations of this study, it is not clearly known if the effect could be similar to that of epidural analgesia if appropriate pain control is performed in patients who did not receive epidural analgesia. Therefore, future study is needed for evaluating the effect of epidural analgesia on clinical outcomes after lung cancer surgery.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
2. Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Ann Glob Health. 2019; 85:8.
3. Wang Z, Hu L, Li J, Wei L, Zhang J, Zhou J. Magnitude, temporal trends and inequality in global burden of tracheal, bronchus and lung cancer: findings from the Global Burden of Disease Study 2017. BMJ Glob Health. 2020; 5:e002788.
4. Jones GS, Baldwin DR. Recent advances in the management of lung cancer. Clin Med (Lond). 2018; 18(Suppl 2):S41–6.
5. Bendixen M, Jorgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol. 2016; 17:836–44.
6. Handy JR Jr, Asaph JW, Skokan L, Reed CE, Koh S, Brooks G, et al. What happens to patients undergoing lung cancer surgery? Outcomes and quality of life before and after surgery. Chest. 2002; 122:21–30.
7. Obuchi T, Yoshida Y, Moroga T, Miyahara N, Iwasaki A. Postoperative pain in thoracic surgery: re-evaluating the benefits of VATS when coupled with epidural analgesia. J Thorac Dis. 2017; 9:4347–52.
8. Okuda J, Suzuki T, Wakaizumi K, Kato J, Yamada T, Morisaki H. Effects of thoracic epidural anesthesia on systemic and local inflammatory responses in patients undergoing lung cancer surgery: a randomized controlled trial. J Cardiothorac Vasc Anesth. 2022; 36:1380–6.
9. Ng A, Swanevelder J. Pain relief after thoracotomy: is epidural analgesia the optimal technique? Br J Anaesth. 2007; 98:159–62.
10. Dziedzic D, Orlowski T. The role of VATS in lung cancer surgery: current status and prospects for development. Minim Invasive Surg. 2015; 2015:938430.
11. Steinthorsdottir KJ, Wildgaard L, Hansen HJ, Petersen RH, Wildgaard K. Regional analgesia for video-assisted thoracic surgery: a systematic review. Eur J Cardiothorac Surg. 2014; 45:959–66.
12. Luchtenborg M, Riaz SP, Coupland VH, Lim E, Jakobsen E, Krasnik M, et al. High procedure volume is strongly associated with improved survival after lung cancer surgery. J Clin Oncol. 2013; 31:3141–6.
13. Freise H, Van Aken HK. Risks and benefits of thoracic epidural anaesthesia. Br J Anaesth. 2011; 107:859–68.
14. Goto T. What is the best pain control after thoracic surgery? J Thorac Dis. 2018; 10:1335–8.
15. Giebler RM, Scherer RU, Peters J. Incidence of neurologic complications related to thoracic epidural catheterization. Anesthesiology. 1997; 86:55–63.
16. Kim JA, Kim TH, Yang M, Gwak MS, Kim GS, Kim MJ, et al. Is intravenous patient controlled analgesia enough for pain control in patients who underwent thoracoscopy? J Korean Med Sci. 2009; 24:930–5.
17. Ding X, Jin S, Niu X, Ren H, Fu S, Li Q. A comparison of the analgesia efficacy and side effects of paravertebral compared with epidural blockade for thoracotomy: an updated meta-analysis. PLoS One. 2014; 9:e96233.
18. Popping DM, Elia N, Marret E, Remy C, Tramer MR. Protective effects of epidural analgesia on pulmonary complications after abdominal and thoracic surgery: a meta-analysis. Arch Surg. 2008; 143:990–9.
19. Licker M, Spiliopoulos A, Frey JG, Robert J, Hohn L, de Perrot M, et al. Risk factors for early mortality and major complications following pneumonectomy for non-small cell carcinoma of the lung. Chest. 2002; 121:1890–7.
20. Simonsen DF, Sogaard M, Bozi I, Horsburgh CR, Thomsen RW. Risk factors for postoperative pneumonia after lung cancer surgery and impact of pneumonia on survival. Respir Med. 2015; 109:1340–6.
21. Wu HL, Tai YH, Chan MY, Tsou MY, Chen HH, Chang KY. Effects of epidural analgesia on cancer recurrence and long-term mortality in patients after non-small-cell lung cancer resection: a propensity score-matched study. BMJ Open. 2019; 9:e027618.
22. Xu ZZ, Li HJ, Li MH, Huang SM, Li X, Liu QH, et al. Epidural anesthesia-analgesia and recurrence-free survival after lung cancer surgery: a randomized trial. Anesthesiology. 2021; 135:419–32.
Fig. 1.
The trends of epidural analgesia for lung cancer surgery from 2011 through 2018 in South Korea.
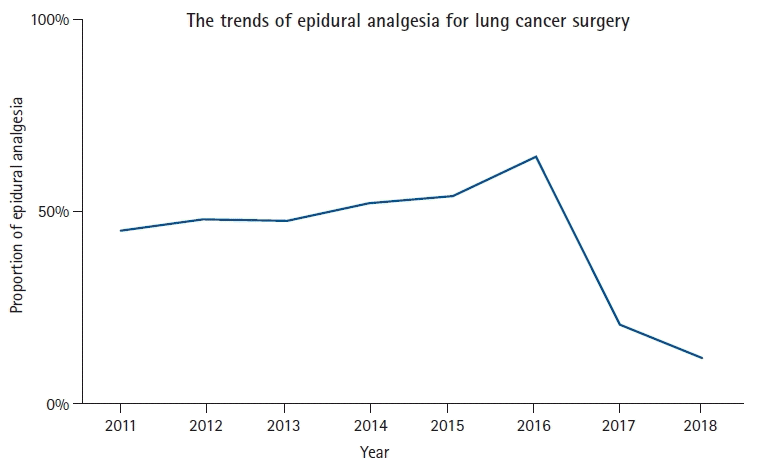
Table 1.
Clinicopathological Characteristics of the Patients
Table 2.
Comparison of Clinicopathological Characteristics between the Epidural Analgesia and Control Groups
Table 3.
Multivariate Logistic Regression Model for the Application of Epidural Analgesia
Table 4.
Analyses regarding 30-day Mortality, Fatal Respiratory Events, and One-year Mortality Associated with Epidural Analgesia for Lung Cancer Surgery




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download