Abstract
In the highly contagious coronavirus disease 2019 pandemic, aerosol-generating procedures (AGPs) are associated with high-risk of transmission. Upper gastrointestinal endoscopy is a procedure with the potential to cause dissemination of bodily fluids. At present, there is no consensus that endoscopy is defined as an AGP. This review discusses the current evidence on this topic with additional management. Prevailing publications on coronavirus related to upper gastrointestinal endoscopy and aerosolization from the PubMed and Scopus databases were searched and reviewed. Comparative quantitative analyses showed a significant elevation of particle numbers, implying that aerosols were generated by upper gastrointestinal endoscopy. The associated source events have also been reported. To reduce the dispersion, certain protective measures have been developed. Endoscopic unit protocols are recommended for the concerned personnel. Therefore, upper gastrointestinal endoscopy should be classified as an AGP. Proper practices should be adopted by healthcare workers and patients.
Since the global outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, over 400 million patients have been infected. Due to the high-risk of human-to-human transmission via aerosols and droplets, upper gastrointestinal endoscopy was initially withheld because of the potential to release aerosols from the oropharynx by the gag reflex or coughing or from body fluids, especially at less than 1 m from the patient. Thus, preprocedural polymerase chain reaction (PCR) for the coronavirus disease 2019 (COVID-19) virus was performed in patients who required an endoscopy. Personal protective equipment (PPE) with an N95 respirator was mandated in all procedures on patients with the infection. The endoscopic unit was prepped before the procedure and the disinfection of instruments was required after completion. Non-urgent cases were deferred to reserve necessary resources. This caused a delay in diagnosis and management. The endoscopic case volumes decreased by 50% to 80%.1 The estimated reduction in the detection of intraluminal gastrointestinal malignancies was over 20%, which may lead to cancer-related crises in the future. Our review aims to assess the possibility of aerosol generation during upper gastrointestinal endoscopy. Appropriate peri-procedural management is being explored to mitigate further infection to healthcare workers (HCW), and to reduce the delay in endoscopic diagnosis and treatment for patients in the pandemic.
SARS-CoV-2 can be detected in oropharyngeal swab specimens, blood, saliva, urine, and feces. Previous studies on isolated viral RNA by real-time PCR have confirmed the involvement of multiple organs in the infection. The infection is transmitted frequently via droplets, airborne carriers, and fomites.2-4 After coughing or talking, droplets from the respiratory tract containing viable viruses can infect other individuals. Large droplets of over 5 μm can deposit by falling on surfaces and cause propagation through fomites. During normal breathing, aerosol particles smaller than 5 μm can disperse farther by airborne transmission than by droplets.5 According to the World Health Organization, aerosol-generating procedures (AGPs) includes open airway suction, sputum induction, cardiopulmonary resuscitation, endotracheal intubation and extubation, non-invasive ventilation such as bilevel positive airway pressure and continuous positive airway pressure, bronchoscopy, and manual ventilation. Nebulizer administration and high-flow oxygen delivery are also considered AGPs, but there are limited data available on these.6-8 Several factors are associated with a greater risk of dissemination. Forced air over the respiratory mucosa, such as during coughing and positive-pressure ventilation, can emit virus-laden particles. Severe and symptomatic patients in close-range contact are likely to transmit the infection to others. In poorly ventilated spaces, infected particles may accumulate and be transmitted over a wider area. A prolonged exposure time also results in a higher rate of infection.7
Endoscopy was deemed as an additional aerosolization procedure in described lists.8 Some numerical statistical studies were conducted to prove this theory.9-12 Aerosols from patients undergoing upper gastrointestinal endoscopy were measured by a handheld optical particle counter before, during, and after the procedure. Particle sizes were reported to be in the range of 0.3 to 10 μm.10-12 Sagami et al.9 compared conscious sedated upper gastrointestinal endoscopy with non-endoscopy as a control group, in the same plastic enclosure and positive-pressure environment. They reported a significant increase in the number of particles during and after the procedure in the endoscopy group. A higher body mass index and more episodes of burping might have induced more aerosol generation, according to their multivariate analysis.9 Chan et al.10 also described a significant increase in particles of all sizes during upper gastrointestinal endoscopy, when measured at 10 cm from the patient’s mouth. Application of a dental sucker may reduce the number of counted particles. Gregson et al.11 evaluated the surgical assessment endoscopy of bariatric patients and found that endoscopic-evoked coughs generated more aerosols than the baseline tidal breathing and volitional cough. From these observations, it can be concluded that coughing is a dominant source of aerosols during upper gastrointestinal endoscopy. Compared to trans-nasal endoscopy and lower gastrointestinal endoscopy, per-oral gastroscopy seemed to generate the greatest number of aerosols. The largest particle-generating events were associated with the application of a local anesthetic throat spray, which produced 10 times the particles generated from cough. The second largest event was extubation.12 Considering these prospective quantitative studies, upper gastrointestinal endoscopy should be determined as an AGP. Periprocedural management should follow the AGP protocols to prevent the transmission of infected particles.
Various articles have proposed new inventions of protective devices for use during endoscopic procedures to reduce the spread of aerosols from patients to endoscopic staff. Lai13 developed an aerosol box made from acrylic or transparent polycarbonate sheet, to assist in tracheal intubation during the early COVID-19 outbreak. Two holes were created to fit the insertion of both hands. During intubation, the box was placed to cover the head of the patient. An extra plastic drape could be attached to the uncovered side of the box. This minimized droplet scattering to others. It was reusable after cleaning with alcohol or bleach, and affordable. In the past year, several designs were adapted, resulting in an endoscopic box shield being used in upper gastrointestinal endoscopy. Two holes were added for both hands, and smaller holes were made on the other side of the box for endoscopic shaft introduction (Fig. 1A). Prior studies have proven its protective efficacy by reducing the extent of ultraviolet visualization of fluorescent dye scattered after bursting from a rubber balloon placed in a mannequin’s hypopharynx. Contamination was decreased on the endoscopist’s clothing and the floor of the room.14-17
Another shielding instrument with a more compact feature is the acrylic face shield developed by the Colombia University endoscopic unit.18 They created an open-and-close system orifice for the patient’s mouth. The face shield is worn while waiting and during the procedure. Endoscopy can be performed as usual by opening the orifice (Fig. 1B). Acrylic face shields are intended to be reusable and can be sterilized before they are used on different patients. Moreover, their mass production could reduce manufacturing costs.
Transparent plastic sheets can be modified to be used as less rigid endoscopic barriers. Sabbagh et al.19 invented this by folding a 75×75-cm size plastic sheet with adhesive tape placed away from the edge of the fold to form a slit, and lubricated the opening to facilitate the introduction of the scope. After the oxygen nasal cannula and oral protector are given to the patient, the plastic sheet is fixed to cover the patients head, and endoscopy can be performed by insertion via the created channel (Fig. 1C).
Devices with an entire facial cover may cause patient discomfort. With increased availability and low cost, a double surgical mask with a slit was designed by Lazaridis et al.20 Two surgical masks were taped at the upper and lower borders of the masks, with a 1.2-cm slit cut through both masks using scissors, for insertion of the scope (Fig. 1D). They also applied this clinical practice for antegrade double-ballon enteroscopy.20
Recently, Fujifilm released two endoscopic accessories to reduce the risk of droplet dissemination. One of them is a droplet-shielding mouthpiece called “B1”, which consists of a drape shield to cover the patient’s face to protect HCWs and is attached with double-layer sponges with slits to reduce the gap between the scope and the mouthpiece (Fig. 1E). They have claimed that B1 could eliminate over 99% of droplets 5 μm in size and larger, compared to the currently existing mouthpiece. The second is an endoscope cover called “P1” which covers the forceps valve. The drape sheet covers the entire working portion of the endoscope. Installing P1 to the port of the working channel may minimize secretion spillage when the instrument is pulled out (Fig. 1F).21
In collaboration with Olympus Medical System and an otolaryngologist, Kikuchi et al.22 proposed the shielding device for endoscopic procedures (STEP) as a barrier device. It consists of a mask with a suction port and a valve-like hole for endoscopy, which connects to the vinyl tubular plastic drape taped to the base of the endoscope. During the procedure, the endoscope is stored in the drape and inserted through the mask so that the endoscopist would not be in direct contact with the patient’s aerodigestive fluids (Fig. 1G). An analysis of contact, droplet, and aerosol infections was performed by comparing STEP and non-STEP groups using simulation models. A significantly lower spread was found in all three models with similar procedural times and visual analog scale scores for endoscopic image evaluation from the pharynx to the second part of the duodenum. Therefore, the STEP may be useful for infection control during endoscopy. The summarized information on the tools is shown in Table 1.13-22
The indications for upper gastrointestinal endoscopy should be prioritized as emergency, urgency, or elective procedures.23-25 Temporarily postponed procedures are performed according to the availability of resources. Situations in which procedures should always be performed are unstable gastrointestinal hemorrhage or anemia, upper gastrointestinal foreign body ingestion, obstructive jaundice, and acute cholangitis according to the European Society of Gastrointestinal Endoscopy guidelines.24 Endoscopy in cancer-related, severely symptomatic patients and those who might rapidly deteriorate if deferred should also be performed or postponed for no longer than 12 weeks.
Risk stratification using a questionnaire of symptoms and contacts should be performed in all cases at least 24 to 72 hours prior to endoscopy, and upon arrival to the endoscopic unit. There are no current recommendations on routine testing for SARS-CoV-2, and it should be individualized based on institutional protocols. If necessary, testing should be performed using standard nucleic acid testing with rapid reverse transcription PCR or a nucleic acid amplification test.1 At our center, routine reverse transcription PCR testing was performed in all patients before endoscopic procedures to protect HCWs, and full PPE sets and N95 respirators were reserved for positive or high-risk patients. High-risk patients classified by the presence of respiratory tract symptoms, previous travel to COVID-19 locations in the past 14 days, and close contact with positive patients, should prompt removal from the holding area and self-quarantine. Consent for the procedure must be obtained, and the patient should be informed of the possible risk of COVID-19 infection from undergoing endoscopy. All patients should wear surgical masks and keep at least 1 to 2 m of distance from others. If possible, barriers, such as glass or face shields, should be worn. Temperature measurements and telephone number records were collected prior to entering the endoscopic unit. The patient’s relatives or caregiver should wait offsite and return after the procedure is completed. If this is not feasible, the waiting area should be appropriately distanced.23,25
All endoscopy team members should wear surgical masks, gloves, hair coverings, face shields or goggles, water-proof disposable gowns, and shoe covers or boots. The staff should fill in the same questionnaire as patients before their daily work. Similar distancing should be maintained between individuals. Arrangements made in advance could reduce patient congestion in the waiting area. Chairs and beds must be spaced to avoid the transmission of viral particles to non-infected patients. Virucidal agents are recommended for cleaning and disinfection. The routine use of disposable endoscopic devices is not recommended. Sufficient hand washing with soap or alcohol-based solutions, before and after any contact with potential viral sources, can reduce transmission to others. For positive cases, the procedure should be performed in a negative-pressure endoscopic unit, if available. An alternative is using a portable industrial-grade high-efficiency particulate air filter. In low-resource situations, adequate ventilation of the room is acceptable.26,27
PPE for endoscopy personnel should be adjusted according to patient risk stratification. A full PPE is essential for high-risk or confirmed positive patients. The PPE includes a filtering facepiece 2 or 3 mask, double gloves, and other coverings, as mentioned above. In low-resource settings, reusable respirators, face shields, goggles, and boots may be acceptable after appropriate sterilization and decontamination methods.26 The staff should be trained in the correct sequence of donning and doffing the PPE, as it is critical. A minimal number of workers should be in the room and the team should not be switched during procedures to minimize PPE usage and decrease the contamination risk. A bedside endoscopy can be performed for critically ill patients in the intensive care unit. Patients with persistent cough should remain in the room until it subsides. After completion of the procedure, the patient must be transported to the recovery area as soon as deemed safe. Procedure-related staff should properly remove the PPE and wash their hands. As much as possible, all required documentation should be done outside the room.
After a COVID-19 patient is shifted out of the procedure room, the room should be maintained under negative pressure for at least 30 minutes, and in the absence of negative pressure, for 60 minutes, before the next patient, to allow for the dispersion of virus-containing aerosols. Standard cleaning with a hospital-grade disinfectant solution should be done by personnel wearing PPE. Reprocessing staff should also wear PPE due to the risk of exposure to contaminated devices. All patients should be traced and contacted after 7 and 14 days, to enquire about new COVID-19 diagnoses or symptoms.23,28
An upper gastrointestinal endoscopy protocol in the current COVID-19 pandemic should be provided, as the procedure is considered an AGP. Periprocedural preparation and protective devices are necessary to prevent the aerosol dispersion of infection, which would be beneficial for healthcare personnel and patients.
REFERENCES
1. Sultan S, Siddique SM, Singh S, et al. AGA rapid review and guideline for SARS-CoV2 testing and endoscopy post-vaccination: 2021 update. Gastroenterology. 2021; 161:1011–1029.
2. Kutti-Sridharan G, Vegunta R, Vegunta R, et al. SARS-CoV2 in different body fluids, risks of transmission, and preventing COVID-19: a comprehensive evidence-based review. Int J Prev Med. 2020; 11:97.
3. Peng L, Liu J, Xu W, et al. SARS-CoV-2 can be detected in urine, blood, anal swabs, and oropharyngeal swabs specimens. J Med Virol. 2020; 92:1676–1680.
4. Taweerutchana V, Suwatthanarak T, Methasate A, et al. Laparoscopic surgery produced less surgical smoke and contamination comparing with open surgery: the pilot study in fresh cadaveric experiment in COVID-19 pandemic. BMC Surg. 2021; 21:422.
5. Rana SS. Risk of COVID-19 transmission during gastrointestinal endoscopy. J Dig Endosc. 2020; 11:27–30.
6. Tran K, Cimon K, Severn M, et al. Aerosol-generating procedures and risk of transmission of acute respiratory infections: a systematic review. CADTH Technol Overv. 2013; 3:e3201.
7. Klompas M, Baker M, Rhee C. What is an aerosol-generating procedure? JAMA Surg. 2021; 156:113–114.
8. Centers for Disease Control and Prevention. Which procedures are considered aerosol generating procedures in healthcare settings? [Internet]. Atlanta (GA): Centers for Disease Control and Prevention;2021. [cited 2022 Jan 11]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/faq.html.
9. Sagami R, Nishikiori H, Sato T, et al. Aerosols produced by upper gastrointestinal endoscopy: a quantitative evaluation. Am J Gastroenterol. 2021; 116:202–205.
10. Chan SM, Ma TW, Chong MK, et al. A proof of concept study: esophagogastroduodenoscopy is an aerosol-generating procedure and continuous oral suction during the procedure reduces the amount of aerosol generated. Gastroenterology. 2020; 159:1949–1951.
11. Gregson FKA, Shrimpton AJ, Hamilton F, et al. Identification of the source events for aerosol generation during oesophago-gastro-duodenoscopy. Gut. 2021; 71:871–878.
12. Phillips F, Crowley J, Warburton S, et al. Aerosol and droplet generation in upper and lower gastrointestinal endoscopy: whole procedure and event-based analysis. Gastrointest Endosc. 2022; 96:603–611.
13. Lai HY. Aerosol box [Internet]. 2020 [cited 2022 Jan 10]. Available from: https://sites.google.com/view/aerosolbox/home?authuser=0.
14. Sagami R, Nishikiori H, Sato T, et al. Endoscopic shield: barrier enclosure during the endoscopy to prevent aerosol droplets during the COVID-19 pandemic. VideoGIE. 2020; 5:445–448.
15. Campos S, Carreira C, Marques PP, et al. Endoprotector: protective box for safe endoscopy use during COVID-19 outbreak. Endosc Int Open. 2020; 8:E817–E821.
16. Charoenwat B, Sirirattanakul S, Hangnak K, et al. “Endoshield”: a physical protective box for pediatric endoscopy during the COVID-19 pandemic. Clin Endosc. 2021; 54:688–693.
17. Kitiyakara T, Kamalaporn P, Poolsombat A, et al. Leak-testing of an endoscopic aerosol box for preventing SARS-CoV-2 infection during upper gastrointestinal endoscopy. Siriraj Med J. 2021; 73:702–709.
18. Aponte Martín DM, Corso C, Fuentes C, et al. Use of a new face shield for patients of the endoscopy unit to avoid aerosol exchange in the COVID-19 era. VideoGIE. 2020; 5:522–524.
19. Sabbagh L, Huertas M, Preciado J, et al. New protection barrier for endoscopic procedures in the era of pandemic COVID-19. VideoGIE. 2020; 5:614–617.
20. Lazaridis N, Skamnelos A, Murino A, et al. “Double-surgical-mask-with-slit” method: reducing exposure to aerosol generation at upper gastrointestinal endoscopy during the COVID-19 pandemic. Endoscopy. 2020; 52:928–929.
21. Fujifilm Corporation. Fujifilm launches droplet-shielding mouthpiece “B1” and endoscope cover “P1” [Internet]. Tokyo: Fujifilm Corp;2020. [cited 2021 Dec 25]. Available from: https://www.fujifilm.com/jp/en/news/hq/5477.
22. Kikuchi D, Ariyoshi D, Suzuki Y, et al. Possibility of new shielding device for upper gastrointestinal endoscopy. Endosc Int Open. 2021; 9:E1536–E1541.
23. Gralnek IM, Hassan C, Beilenhoff U, et al. ESGE and ESGENA Position Statement on gastrointestinal endoscopy and the COVID-19 pandemic. Endoscopy. 2020; 52:483–490.
24. South African Gastroenterology Society (SAGES). SAGES position statement on endoscopy and PPE requirements during COVID-19 pandemic [Internet]. Mowbray: SAGES;2020. [cited 2022 Jan 3]. Available from: https://www.sages.co.za/newsletter_SAGES/PPE_Requirements.pdf.
25. Hennessy B, Vicari J, Bernstein B, et al. Guidance for resuming GI endoscopy and practice operations after the COVID-19 pandemic. Gastrointest Endosc. 2020; 92:743–747.
26. Leddin D, Armstrong D, Raja Ali RA, et al. Personal protective equipment for endoscopy in low-resource settings during the COVID-19 pandemic: guidance from the World Gastroenterology Organisation. J Clin Gastroenterol. 2020; 54:833–840.
27. Kongkam P, Tiankanon K, Ratanalert S, et al. The practice of endoscopy during the COVID-19 pandemic: recommendations from the Thai Association for Gastrointestinal Endoscopy (TAGE) in collaboration with the Endoscopy Nurse Society (Thailand). Siriraj Med J. 2020; 72:283–286.
28. Antonelli G, Karsensten JG, Bhat P, et al. Resuming endoscopy during COVID-19 pandemic: ESGE, WEO and WGO Joint Cascade Guideline for resource limited settings. Endosc Int Open. 2021; 9:E543–E551.
Fig. 1.
Illustration of tools for preventing aerosols from upper gastrointestinal endoscopy. (A) Acrylic box. (B) Acrylic face shield. (C) Plastic sheet. (D) Double surgical mask. (E) B1. (F) P1. (G) Shielding device for endoscopic procedures.
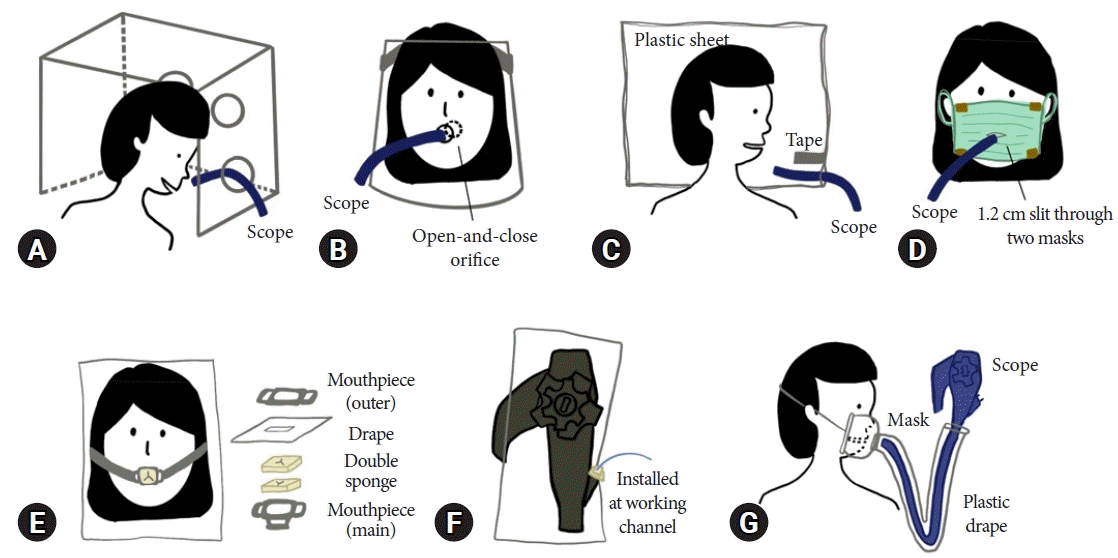
Table 1.
Tools for protection from aerosols
| Device (year) | Reusable | Availability | Comfortable | Cost | Effectiveness |
|---|---|---|---|---|---|
| Acrylic box (2020)13-17 | Yes | Producible | Yes | 67 US$ | Yes (fluorescent dye) |
| Acrylic face shield (2020)18 | Yes | Producible | No | 5 US$ | NA |
| Plastic sheet (2020)19 | No | Yes | No | Low | NA |
| Double surgical mask (2020)20 | No | Yes | Yes | Low | NA |
| B1 and P1 (2020)21 | No | Developed by Fujifilm | Yes | NA | 99% for droplets tested in B1 |
| STEP (2021)22 | No | Collaborated with Olympus | No | NA | 99% (reduced particles) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download