Abstract
Background/Aims
Endoscopic submucosal dissection (ESD) for residual or recurrent colorectal lesions after incomplete resection is challenging because of severe fibrosis. This study aimed to compare the efficacy of the pocket-creation method (PCM) with a traction device (TD) with that of conventional ESD for residual or recurrent colorectal lesions.
Methods
We retrospectively studied 72 patients with residual or recurrent colorectal lesions resected using ESD. Overall, 31 and 41 lesions were resected using PCM with TD and conventional ESD methods, respectively. We compared patient background and treatment outcomes between the PCM with TD and conventional ESD groups, respectively. The primary endpoints were en bloc resection and R0 resection rates. The secondary endpoints were the dissection speed and incidence of adverse events.
Results
En bloc resection was feasible in all cases with PCM with TD, but failed in 22% of cases of conventional ESD. The R0 resection rates for PCM with TD and conventional ESD were 97% and 66%, respectively. Dissection was significantly faster in the PCM with TD group (13.0 vs. 7.9 mm2/min). Perforation and postoperative bleeding were observed in one patient in each group.
Endoscopic mucosal resection (EMR) is a standard technique used for colorectal tumor resection, especially in Western countries, because of its simplicity and medical economy.1 However, it has uncertain outcomes for lesions measuring ≥20 mm and submucosal invasive cancer. The occurrence of residual or recurrent lesions due to incomplete resection after EMR/endoscopic piecemeal mucosal resection (EPMR) is problematic.2 The advent of endoscopic submucosal dissection (ESD) has made en bloc and R0 resection of colorectal tumors possible, with almost no recurrence, irrespective of tumor size.1,3,4 However, residual or recurrent lesions after incomplete resection by EMR/EPMR are associated with insufficient lifting with local injection due to severe fibrosis, and ESD for these lesions is extremely technically demanding, even for experts.5,6
Various techniques have been reported for the endoscopic treatment of residual or recurrent colorectal lesions. Ablation techniques such as snare tip and argon plasma coagulation are simple and easy to use.7 However, the main concern is that tissue specimens cannot be collected for pathological diagnosis. The avulsion technique is also a simple and easy technique; however, an accurate pathological diagnosis with this technique is difficult because it does not allow complete en bloc resection.7 The grasp-and-snare technique has a higher possibility of accurate pathological diagnosis than the avulsion technique, but it requires a double-channel endoscope, and depending on the lesion size, en bloc resection may be difficult.8,9 Thus, additional surgical resections may be performed for these lesions to ensure accurate pathological evaluations.10
Therefore, an ESD technique that can perform R0 resection endoscopically for residual or recurrent colorectal lesions is needed. We recently reported the pocket-creation method (PCM) with a traction device (TD) (Fig. 1), enabling safe en bloc resection even in difficult situations.11,12 This study aimed to evaluate the efficacy and safety of PCM with TD compared with conventional ESD for residual or recurrent colorectal lesions after incomplete resection by polypectomy or EMR/EPMR.
Seventy-two patients with residual or recurrent colorectal lesions underwent ESD at the Cancer Institute Hospital of the Japanese Foundation for Cancer Research between April 2012 and June 2020. Medical records of all cases were collected consecutively, with no exclusions. We divided the 72 patients into two groups according to the ESD procedure; 41 lesions treated with conventional ESD served as the control group and were compared with 31 lesions in the PCM with TD group. Magnifying endoscopy was performed before ESD for epithelial tumors to increase the accuracy of invasion depth diagnosis. Endoscopic ultrasonography was used when there was concern for submucosal recurrence with a “normal” mucosal appearance.
Recurrent lesions were defined as persistent lesions at the same site that had been previously resected with one or more endoscopic resections (polypectomy or EMR/EPMR). Residual lesions were defined as lesions that were partially resected with one or more endoscopic resections (polypectomy or EMR/EPMR). In addition, cases where the “vertical margin was positive in previously resected pathological findings” were included as residual lesions even if the endoscopic findings revealed only scarring and no tumors on the mucosal surface. The reason for this classification is that it is impossible to rule out residual tumors in the submucosal layer.
The ESD procedure was performed as previously described.12 The patients were administered 1,800 mL Magcorol P (68 g of magnesium citrate; Horii Pharmaceutical, Osaka, Japan) the day before ESD as pretreatment. On the day of ESD, 2 L of oral polyethylene glycol solution was administered. Midazolam and pethidine hydrochloride were administered intravenously for conscious sedation. Hyoscine-N-butyl bromide or glucagon was administered intravenously as antispasmodics. For the endoscope, GIF-Q260J and GIF-H290T were used for rectal or anal colonic lesions, and PCF-Q260J and PCF-H290T were used for oral colonic lesions. A disposable hood with a wide tip opening diameter (D-201-11804; Olympus, Tokyo, Japan) was used for attachment. We used a dual knife (KD-650 Q; Olympus) or dual knife J (KD 655 Q; Olympus). Hemostatic forceps (FD-411QR; Olympus) were used when arterial bleeding was difficult to control using the knife tip. The VIO 300 D (ERBE Elektromedizin GmbH, Tubingen, Germany) was used as the high-frequency device, and mucosal incisions and most of the submucosal dissections were made in the Endo-Cut I mode (effect, 2; duration, 2; and interval, 2), and the SWIFT coagulation mode (effect 3, 45 W) was used to dissect the submucosa with fat tissue and a large number of blood vessels. Carbon dioxide was used in all cases. Submucosal injection of glycerin and 0.4% sodium hyaluronate mixed with a small amount of indigo carmine and epinephrine was administered to elevate the submucosa.
Figure 2 shows the schematic of each method. Conventional ESD was the only procedure performed in the early part of the study period, between April 2012 and June 2016. Since we developed a PCM with TD in July 2016, PCM with TD has been applied to most procedures. The TD used in this study was created with a ring-shaped nylon thread with a diameter of approximately 15 mm and attached to the base of the endoscopic clip (HX-610-090S; Olympus). TD was delivered through the scope, and the traction force was adjusted by the volume of air supply in the lumen, and there were no restrictions on the location of the lesion or the direction of traction.
The PCM with TD procedure was performed as follows. (1) Incision and dissection were performed on the oral side of the lesion, as in conventional ESD. (2) Sufficient local injection was performed around the lesion on the anal side in the forward position. Before starting the incision on the anal side, the TD was attached approximately 10 mm from the lesion on the anal side. An incision was made anal to the TD, and traction was applied to the superficial mucosal plane. The surrounding mucosa and submucosal layer to which the TD was attached were immediately under sufficient countertraction, which easily exposed the submucosal layer once the incision was made. (3) Following a few cuts of the submucosal layer, a mucosal flap was formed, facilitating the entry of the endoscope tip. A submucosal pocket was easily created during the initial dissection process. Even in sites with severe fibrosis, the locally infused solution was less prone to diffuse because of the absence of a circumferential incision. Moreover, the dissection line was easily identified because two traction forces were created in the submucosal pocket by hood attachment and TD. A circumferential incision was made once the submucosal pocket exceeded 50% of the lesion area beyond the center of the lesion. Dissection was then performed using the same approach as that used for conventional ESD (Fig. 3, Supplementary Video 1).
Experts were defined as endoscopists with experience in handling 100 or more cases of colorectal ESD before the study period. Trainees were defined as endoscopists with experience in managing 500 or more colonoscopies and fewer than 10 cases of colorectal ESD prior to the start of the study period, assuming accurate diagnostic ability using magnifying endoscopy and skill in EMR. All ESD procedures performed by the trainees were performed under the supervision of an expert. Supplementary Table 1 shows the breakdown of the trainee and expert endoscopists included in this study. A total of 72 procedures were performed by five experts and 15 trainees.
If the lesion was resected in a single piece, resection was defined as en bloc resection. The lesion was defined as R0 if the horizontal and vertical margins were negative on pathological examination after en bloc resection. The size of the specimen, procedure time, and pathological findings were evaluated after the resection was completed. The area (mm2) of the resected specimen was calculated using the following formula:
Area = large diameter (mm)/2 × small diameter (mm)/2 × 3.14
Procedure time was defined as the time from the first local injection into the submucosa to complete resection of the lesion. The dissection speed (mm2/min) was calculated using the area of the resected specimen (mm2)/dissection time (min).
Fibrosis was assessed intraoperatively in accordance with previously reported definitions.13 No fibrosis was defined as F0, mild fibrosis as F1, and whitish submucosa or severe fibrosis as F2.
Perforation was defined as a complete hole through the muscle layer during the procedure or clinical evidence of air in the abdominal cavity on postoperative computed tomography. Bleeding was defined as bloody stool requiring endoscopic hemostasis within 2 weeks of ESD.
Comparisons between two groups were performed using the Mann-Whitney U-test for continuous variables. Categorical variables were compared using chi-squared and Fisher exact tests. Logistic regression analysis was performed on the factors affecting the R0 resection rate, including methods (conventional ESD or PCM with TD) and operator (trainee or expert) by preceding studies. The factors previously reported to be significantly associated with technical difficulties in colorectal ESD included lesion size and lesion location.14,15 All analyses were performed using R ver. 3.4.2 (R Foundation, Vienna, Austria), and a p-value of <0.05 was considered statistically significant.
All procedures were performed in accordance with the 1964 Declaration of Helsinki and its later amendments. This study was approved by the ethics committee of the Institutional Review Board of the Cancer Institute Hospital of the Japanese Foundation for Cancer Research (IRB No: 2021-GB-038), and the requirement for written informed consent from the patients was waived due to the retrospective nature of the study.
The patient characteristics are shown in Table 1. There were fewer cases of rectal lesions in the PCM with TD group than in the control group (23% [7/31] vs. 51% [21/41], p=0.047). The percentage of ESD performed by trainees was significantly higher in the PCM with TD group than in the control group (68% [21/31] vs. 29% [12/41], p=0.001). There were no significant differences in age, sex, lesion morphology, fibrosis degree, or lesion size between the two groups. In the latter half of the survey period, PCM with TD became the mainstream procedure, and the use of conventional ESD decreased after introducing PCM with TD (Supplementary Table 2).
The treatment outcomes are presented in Table 2. TD was useful in all cases. All 31 lesions in the PCM with TD group were resected en bloc, while 22% (9/41) of the control group failed to undergo en bloc resection (p=0.008). The R0 resection rate was also significantly higher in the PCM with TD group than in the control group (97% [30/31] vs. 66% [27/41], p=0.001). The dissection speed was significantly higher in the PCM with TD group than in the control group (13.0 mm2/min vs. 7.9 mm2/min, p=0.002). We conducted a multivariate analysis to identify the factors affecting the ESD completion rate (Table 3). Performing PCM with TD was identified as a significant positive factor for achieving R0 resection (odds ratio, 10.40; 95% confidence interval, 1.11–98.50; p=0.040). The characteristics of patients without R0 resection are shown in Table 4. Of the 14 patients who did not undergo R0 resection after conventional ESD, 11 were positive for vertical margins, which affected the accurate final histopathological evaluation. However, in all cases of PCM with TD, resection was performed with a negative vertical margin.
In terms of adverse events, there was no significant difference between groups with respect to perforation or postoperative bleeding rates (2.4% [1/41] vs. 3.2% [1/31], p=1.00; 2.4% [1/41] vs. 3.2% [1/31], p=1.00). Both cases of perforation were minor that occurred during ESD. In both cases, the perforation sites were completely closed with endo-clips. The cases were conservatively managed with antibiotics, and none of the patients required emergency surgery.
PCM with TD is a simple method but has the great advantage of addressing challenging technical issues related to severe fibrosis occurring after incomplete resection in initial endoscopic treatment by ensuring a good visual field and determining the appropriate dissection line. This is the first report showing the clinical utility of PCM with TD for residual or recurrent colorectal lesions. Using PCM with TD, all the lesions could be resected en bloc, and all but one had an R0 resection, whereas 22% and 34% failed to undergo en bloc and R0 resection in the control group, respectively. In particular, conventional ESD has a high rate of positive vertical margins, which can be avoided using our PCM with TD method, which may lead to a more accurate final pathological evaluation in these cases. An accurate final pathological evaluation with a negative vertical margin is essential for risk assessment, including the possibility of local as well as lymph node recurrence, and is an important factor in determining additional surgical resection in patients.
En bloc resection rate of residual or recurrent lesions with conventional ESD has been reported to be not favorable (56%–96%).16-19 However, using PCM with TD, we have shown excellent results with 100% en bloc resection rate and 97% R0 resection rate. In terms of perforation, only one case of minor perforation was observed during ESD, which did not require emergency surgery. Furthermore, all previous reports on ESD for residual or recurrent lesions were single-arm studies with no comparison group. This study demonstrates the use of PCM with TD for achieving safe and reliable ESD for residual or recurrent lesions, with conventional ESD as the control group.
The usefulness of TD alone for residual or recurrent colorectal lesions has already been reported.20 A study has shown that TD improves the visibility of the submucosal layer, thereby enabling safe and efficient dissection. However, the report was limited to procedures performed by four skilled experts with experience of 200 or more cases of colorectal ESD. It is difficult even for experts to identify a safe dissection line for severe fibrotic lesions when sufficient lifting cannot be achieved because the local infusion fluid leaks from the submucosa after making a circumferential incision. Advantages of PCM with TD for residual or recurrent lesions that compensate for these shortcomings are as follows: (1) the initial incision is made with the mucosa already under traction, which facilitates the formation of the mucosal flap by securing a sufficient margin on the anal side, enabling the rapid formation of a submucosal pocket; and (2) even if the site has severe fibrosis, a circumferential incision is not made, which makes the local infusion solution less prone to diffusion, and the two traction forces, the hood attachment and TD, are exerted within the submucosal pocket, facilitating identification of the dissection line.12 This excellent visualization reduces the risk of perforation and enables identification of the correct dissection line immediately above the muscle layer, resulting in en bloc resection with a negative vertical margin. Confirmation of a negative vertical margin, even in lesions with severe fibrosis, is paramount in determining whether colorectal resection with lymph node dissection is required.21 Use of PCM with TD provides high-quality ESD and assists in the determination of the next strategy.
A previous systematic review reported that perforation occurred in approximately 7% of ESD procedures, even for lesions without fibrosis.22 Residual or recurrent colorectal lesions are associated with severe fibrosis in the submucosa, making it difficult to visualize the laminar structure of the colorectal wall, and the intraoperative complication rate has been reported to be even higher (14%–32%).16,23 The usefulness of underwater EMR for recurrent lesions has also been reported, but the en bloc resection rate was 47%, which is far below that of PCM with TD.24 Endoscopic full-thickness resection is an alternative method; however, its outcomes are also not sufficiently good (R0 resection rate for residual or recurrent lesions <2 cm, 80%; ≥2 cm, 58.1%; and perforation rate 9.9%).25,26 Reports from Japan have demonstrated the usefulness of laparoscopic and endoscopic cooperative surgery for difficult ESD cases with severe fibrosis, such as residual or recurrent lesions, and good results have been achieved, with an R0 resection rate of 100%.27,28 However, the reports are limited to only a small number of cases, and this procedure has not been established as a standard treatment due to the level of invasiveness, requirement for general anesthesia, and costs.
This study has several limitations. First, this was a retrospective, single-center observational study. Second, there was a timeframe shift between the two groups. There is a possibility that differences in the level of procedural proficiency may have affected the outcome of the treatment, but it should be noted that the outcome of the second half of the study was considerably better than that of the first half, even though most of the ESD procedures were performed by trainees. We also found that ESD performed by experts was not a significant predictor of R0 resection in the multivariate analysis. The control group had more experts than the PCM with TD group; however, the PCM with TD group showed better results than the control group. Therefore, the treatment outcomes were influenced by the dissection method (PCM with TD) used rather than the endoscopist’s experience. In other words, the PCM with TD technique allows trainees to complete the procedure with a good visual field and to identify a proper dissection line. Third, the results of this study only compared the PCM with TD method with the conventional ESD method, and a prospective randomized controlled trial is preferable to verify whether the PCM with TD method is superior to the ESD method with PCM or TD only. However, we believe that the PCM method contributes to the present results, consistent with those that we have already reported on the superiority of the PCM with TD method over the TD method alone in different research participants.
In conclusion, this study showed that PCM with TD is a safe and effective procedure for treating residual or recurrent colorectal lesions occurring after incomplete resection during initial endoscopic treatment. Moreover, we believe that since PCM with TD could also be performed safely by less experienced endoscopists as opposed to conventional ESD, where success was linked to the endoscopist's experience, a wider adoption may help make the treatment readily available.
Supplementary Material
Supplementary Video 1. Pocket-creation method with a traction device for a residual or recurrent lesion in the transverse colon (https://doi.org/10.5946/ce.2022.009.v001).
Supplementary Table 2. Proportion of cases involving the PCM with TD or conventional ESD.
Supplementary materials related to this article can be found online at https://doi.org/10.5946/ce.2022.009.
Notes
Acknowledgements
The authors thank all our exceptional colleagues at the Department of Gastroenterology for the clinical management of the patients.
Author Contributions
Conceptualization: DI, TRO; Data curation: DI, MI; Formal analysis, DI, AN; Investigation: DI, YE, EN, YM, JT, KS, SY, CY; Methodology: DI, MI; Project administration: DI; Supervision: DI, TRO, MI, AC, MI, AN, MS, SS, JF; Validation: DI, TRO, MI, AN; Visualization: DI, TRO, MI; Writing - original draft: DI, TRO, MI; Writing-review & editing: all authors.
REFERENCES
1. Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015; 47:829–854.
2. Oka S, Tanaka S, Kaneko I, et al. Endoscopic submucosal dissection for residual/local recurrence of early gastric cancer after endoscopic mucosal resection. Endoscopy. 2006; 38:996–1000.
3. Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc. 2010; 72:1217–1225.
4. Repici A, Hassan C, De Paula Pessoa D, et al. Efficacy and safety of endoscopic submucosal dissection for colorectal neoplasia: a systematic review. Endoscopy. 2012; 44:137–150.
5. Lee SP, Kim JH, Sung IK, et al. Effect of submucosal fibrosis on endoscopic submucosal dissection of colorectal tumors: pathologic review of 173 cases. J Gastroenterol Hepatol. 2015; 30:872–878.
6. Sato K, Ito S, Kitagawa T, et al. Factors affecting the technical difficulty and clinical outcome of endoscopic submucosal dissection for colorectal tumors. Surg Endosc. 2014; 28:2959–2965.
7. Holmes I, Kim HG, Yang DH, et al. Avulsion is superior to argon plasma coagulation for treatment of visible residual neoplasia during EMR of colorectal polyps (with videos). Gastrointest Endosc. 2016; 84:822–829.
8. von Renteln D, Schmidt A, Vassiliou MC, et al. Endoscopic mucosal resection using a grasp-and-snare technique. Endoscopy. 2010; 42:475–480.
9. de Melo SW Jr, Cleveland P, Raimondo M, et al. Endoscopic mucosal resection with the grasp-and-snare technique through a double-channel endoscope in humans. Gastrointest Endosc. 2011; 73:349–352.
10. Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010; 24:343–352.
11. Ide D, Saito S, Chino A, et al. Submucosal pocket creation using a traction device in colorectal endoscopic submucosal dissection. Ann Gastroenterol. 2018; 31:380.
12. Ide D, Ohya TR, Saito S, et al. Clinical utility of the pocket-creation method with a traction device for colorectal endoscopic submucosal dissection. Surg Endosc. 2021; 35:2110–2118.
13. Matsumoto A, Tanaka S, Oba S, et al. Outcome of endoscopic submucosal dissection for colorectal tumors accompanied by fibrosis. Scand J Gastroenterol. 2010; 45:1329–1337.
14. Takeuchi Y, Iishi H, Tanaka S, et al. Factors associated with technical difficulties and adverse events of colorectal endoscopic submucosal dissection: retrospective exploratory factor analysis of a multicenter prospective cohort. Int J Colorectal Dis. 2014; 29:1275–1284.
15. Imai K, Hotta K, Yamaguchi Y, et al. Preoperative indicators of failure of en bloc resection or perforation in colorectal endoscopic submucosal dissection: implications for lesion stratification by technical difficulties during stepwise training. Gastrointest Endosc. 2016; 83:954–962.
16. Kobayashi R, Hirasawa K, Ikeda R, et al. The feasibility of colorectal endoscopic submucosal dissection for the treatment of residual or recurrent tumor localized in therapeutic scar tissue. Endosc Int Open. 2017; 5:E1242–E1250.
17. Rahmi G, Tanaka S, Ohara Y, et al. Efficacy of endoscopic submucosal dissection for residual or recurrent superficial colorectal tumors after endoscopic mucosal resection. J Dig Dis. 2015; 16:14–21.
18. Zhou P, Yao L, Qin X, et al. Endoscopic submucosal dissection for locally recurrent colorectal lesions after previous endoscopic mucosal resection. Dis Colon Rectum. 2009; 52:305–310.
19. Sakamoto T, Saito Y, Matsuda T, et al. Treatment strategy for recurrent or residual colorectal tumors after endoscopic resection. Surg Endosc. 2011; 25:255–260.
20. Faller J, Jacques J, Oung B, et al. Endoscopic submucosal dissection with double clip and rubber band traction for residual or locally recurrent colonic lesions after previous endoscopic mucosal resection. Endoscopy. 2020; 52:383–388.
21. Takezawa T, Hayashi Y, Shinozaki S, et al. The pocket-creation method facilitates colonic endoscopic submucosal dissection (with video). Gastrointest Endosc. 2019; 89:1045–1053.
22. Thorlacius H, Ronnow CF, Toth E. European experience of colorectal endoscopic submucosal dissection: a systematic review of clinical efficacy and safety. Acta Oncol. 2019; 58:S10–S14.
23. Kuroki Y, Hoteya S, Mitani T, et al. Endoscopic submucosal dissection for residual/locally recurrent lesions after endoscopic therapy for colorectal tumors. J Gastroenterol Hepatol. 2010; 25:1747–1753.
24. Kim HG, Thosani N, Banerjee S, et al. Underwater endoscopic mucosal resection for recurrences after previous piecemeal resection of colorectal polyps (with video). Gastrointest Endosc. 2014; 80:1094–1102.
25. von Helden A, Hildenbrand R, Sido B, et al. Endoscopic full-thickness resection using an over-the-scope device for treatment of recurrent / residual colorectal neoplasia: a single-center case series. BMC Gastroenterol. 2019; 19:121.
26. Schmidt A, Beyna T, Schumacher B, et al. Colonoscopic full-thickness resection using an over-the-scope device: a prospective multicentre study in various indications. Gut. 2018; 67:1280–1289.
27. Fukunaga Y, Tamegai Y, Chino A, et al. New technique of en bloc resection of colorectal tumor using laparoscopy and endoscopy cooperatively (laparoscopy and endoscopy cooperative surgery-colorectal). Dis Colon Rectum. 2014; 57:267–271.
28. Tamegai Y, Fukunaga Y, Suzuki S, et al. Laparoscopic and endoscopic cooperative surgery (LECS) to overcome the limitations of endoscopic resection for colorectal tumors. Endosc Int Open. 2018; 6:E1477–E1485.
Fig. 1.
Procedure for the pocket-creation method with a traction device. (A) Sufficient local injection is performed on the oral side of the lesion, and incision/trimming is performed on approximately half of the lesion circumference. Sufficient local injection is performed on the anal side, and a traction device (TD) is attached approximately 10 mm away from the lesion on the anal side before starting the incision. (B) Another an endoscopic clip is fixed to the attached TD, which is then attached to the contralateral side of the colorectum to the lesion. The surrounding mucosa to which the TD is attached, including the submucosal layer immediately below, is under traction, exposing the submucosal layer as the incision is made. (C) Subsequently, if the submucosal layer is dissected, it forms a mucosal flap, facilitating the entry of the tip of the endoscope. Submucosal pockets rapidly form if dissection is continued without making a circumferential incision. When the submucosal pocket exceeds 50% of the lesion area beyond the center of the lesion, a circumferential incision is made to open the pocket. Then, dissection is performed in stages until it is fully completed.
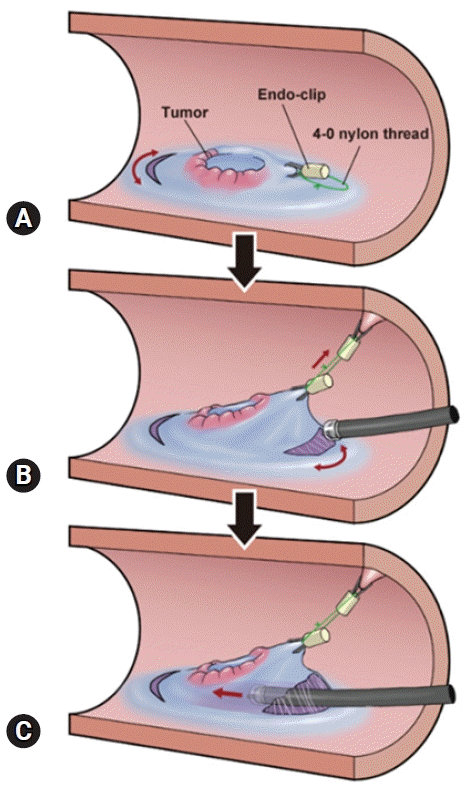
Fig. 2.
Schematic comparison of conventional endoscopic submucosal dissection (ESD) and pocket-creation method (PCM) with a traction device (TD). (A) Conventional ESD: a circumferential incision is made around the lesion, followed by a submucosal incision from the anal side to the oral side. (B) PCM with TD: the key feature of this method is connecting the TD to the anal mucosa 10 mm away from the lesion on the anal side before the initial mucosal incision. The formation of a mucosal flap and creation of a submucosal pocket using the TD becomes easier.
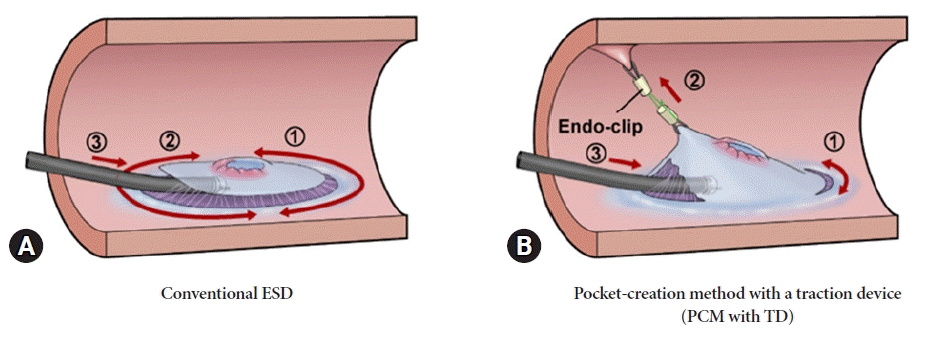
Fig. 3.
Pocket-creation method view with a traction device (TD). (A) A 20-mm lesion in the transverse colon following endoscopic piecemeal mucosal resection. (B) Local injection is performed around the oral lesion, and incision/trimming is performed to approximately one-half of the circumference on the oral side of the lesion. (C) After local injection on the anal side, the TD is attached approximately 10 mm away from the lesion on the anal side. (D) Starting the incision from the anal side of the TD facilitates easy creation of a mucosal flap. (E) A submucosal pocket is formed. (F) Severe fibrosis of the submucosal layer immediately below the lesion scar. (G) Completed circumferential incision and continued dissection. (H) After dissection completion.
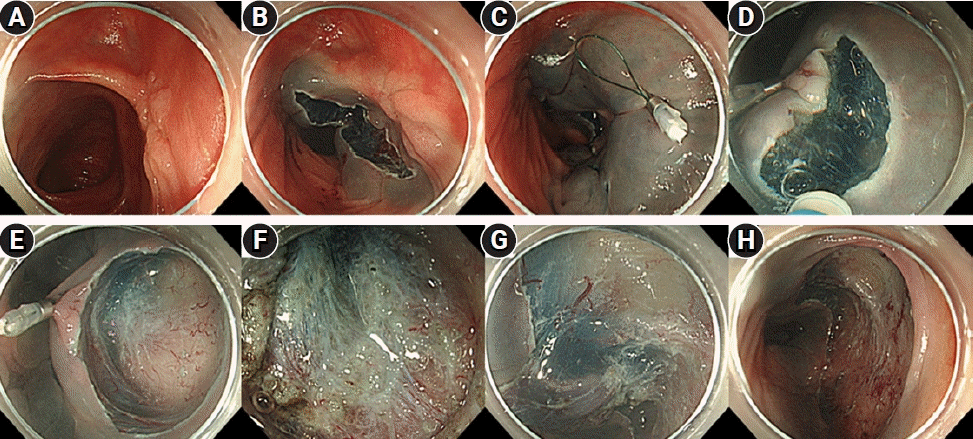
Table 1.
Clinical characteristics of the 72 patients with residual or recurrent colorectal lesions
| Characteristic | Control group (n=41) | PCM with TD group (n=31) | p-value |
|---|---|---|---|
| Age (yr) | 66.0 (57.0–73.0) | 70 (60.0–73.8) | 0.26a) |
| Male sex | 24 (59) | 16 (52) | 0.56b) |
| Location | 0.047b) | ||
| Right colon | 13 (32) | 15 (48) | |
| Left colon | 7 (17) | 9 (29) | |
| Rectum | 21 (51) | 7 (23) | |
| Morphology | 0.11c) | ||
| LST-G | 15 (37) | 4 (13) | |
| LST-NG | 14 (34) | 15 (48) | |
| Flat elevated (<10 mm) | 1 (2.4) | 4 (13) | |
| SMT-like | 4 (9.8) | 3 (9.7) | |
| Scar | 7 (17) | 5 (16) | |
| Fibrosis | 0.92b) | ||
| F0, no fibrosis | 0 | 0 | |
| F1, mild fibrosis | 15 (37) | 11 (36) | |
| F2, whitish submucosa or severe fibrosis | 26 (63) | 20 (65) | |
| Maximum size of the lesion (mm) | 18 (7–25) | 15 (8–25) | 0.99a) |
| Operator, trainee | 12 (29) | 21 (68) | 0.001b) |
Table 2.
A comparison of therapeutic outcomes in the conventional ESD and PCM with TD
| Variable | Control group (n=41) | PCM with TD group (n=31) | p-value |
|---|---|---|---|
| Size of the resected specimen (mm) | 30.0 (20.0–35.0) | 30.0 (30.0–38.5) | 0.076a) |
| Histopathological finding | 0.86b) | ||
| Adenoma-intramucosal cancer | 28 (68.3) | 23 (74.2) | |
| SM slightly invasive cancer (<1,000 μm) | 2 (4.9) | 0 | |
| SM deeply invasive cancer (≥1,000 μm) | 1 (2.4) | 0 | |
| Neuroendocrine tumor | 3 (7.3) | 2 (6.5) | |
| Scar tissue | 7 (17.1) | 6 (19.4) | |
| Procedure time (min) | 82.0 (35.5–76.5) | 56.0 (35.5–76.5) | 0.056a) |
| Dissection speed (mm2/min) | 7.9 (3.1–13.5) | 13.0 (9.2–18.7) | 0.002a) |
| En bloc resection | 32 (78) | 31 (100) | 0.008b) |
| R0 resection | 27 (66) | 30 (97) | 0.001c) |
| Complication | |||
| Perforation | 1 (2.4) | 1 (3.2) | 1.00b) |
| Post-procedural bleeding | 1 (2.4) | 1 (3.2) | 1.00b) |
Table 3.
Logistic regression analysis for R0 resection
Table 4.
Cases of non-R0 resection after ESD for residual or recurrent colorectal lesions




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download