Abstract
Background/Aims
Endoscopic channels are difficult to clean and can cause infection transmission. We examined the effectiveness of a newly developed channel-cleaning ball brush (BB), which is sucked into the endoscopic channel and scrapes and cleans the lumen as it passes through.
Methods
The upper and lower gastrointestinal endoscopes used for patient examinations were randomly selected as the conventional brush (CB) or BB group. After manual cleaning, the presence or absence of carbohydrates, proteins, adenosine triphosphate, and hemoglobin was assessed.
Results
Fifty-six and 58 endoscopes were cleaned with the CB and BB, respectively. Carbohydrate and protein were detected in one (1.8%) and two endoscopes (3.4%) in the CB and BB groups, respectively (p=1.000). Hemoglobin was observed in one (1.8%) and three endoscopes (5.2%) in the CB and BB groups, respectively (p=0.636). The adenosine triphosphate levels were 10.6±15.9 and 12.5±14.3 relative light units in the CB and BB groups, respectively (p=0.496). Twenty-seven (48.2%) and 19 (32.8%) endoscopes were positive for microbial cultures in the CB and BB groups, respectively (p=0.136).
Gastrointestinal endoscopy involves the risk of infection transmission because of direct contact between the endoscope and the patient’s mucous membranes. Furthermore, the mucosal barrier is penetrated when performing procedures such as biopsy, polypectomy, and submucosal dissection.1-3 Infections associated with endoscopy can occur endogenously, from the patient’s own microbiome, but most are exogenous infections from improperly reprocessed equipment, such as endoscopes, endoscopic parts, and reusable endoscopy accessories, which can be vehicles for pathogenic or opportunistic microbes that are transmitted from previous patients.2,4 Meticulous reprocessing of endoscopes is essential to prevent cross infection between patients who undergo endoscopic procedures.5-7
The endoscopic channel is a passage for aspirating various organic materials and specimens in the gastrointestinal tract and inserting accessories of the endoscope, such as biopsy forceps, polypectomy snares, and injection needles. These are likely to leave residue inside the channel, which can be fixed to form a biofilm when appropriate cleaning and rinsing processes are not performed.8,9 When microorganisms are embedded in a biofilm, they are 10–100 times more resistant to cleansing chemicals than planktonic (free-floating) microorganisms and frequently release potentially harmful microbes.4,10,11
The endoscopic channel has a long and narrow structure, making it difficult to inspect and undergo cleaning. In general, the process of reprocessing the endoscope is divided into the steps of manually cleaning the exterior and lumen of the endoscope and disinfecting the endoscope by immersing the endoscope in a high-level disinfectant. In a manual cleaning step, a conventional method to clean endoscopic channels is to manually insert a brush through the channel to scrape off and rinse off debris.5,7,12 This step must be performed completely so that subsequent disinfection steps can be performed effectively. However, this process can be cumbersome and incomplete. It has been found that when brushing with the endoscope shaft bent, the brush preferentially moves along the outer wall of the working channel, and the bristles hardly touch the inside of the curve.13 The cleaning brush can also cause damage to the endoscope in the long term by creating a scratch and shredding inside the channel, which can cause failure of the disinfection process.14
The channel-cleaning ball brush (BB) is a device developed to compensate for this shortcoming. It is made of microfibers wrapped around a silicon sphere, which is sucked into the endoscopic channel and scrapes and cleans the lumen as it passes through. This is a company-sponsored study aimed to compare the efficiency of the BB and conventional brush (CB) in cleaning the channels of gastrointestinal endoscopes.
The study was performed at a secondary referral hospital between August and September 2020. The hospital performs approximately 7,000 upper and 4,000 lower gastrointestinal endoscopies annually, with all relevant personnel having >5 years of experience, completing the reprocessing training and receiving maintenance training every year, supervised by the Korean Society of Gastrointestinal Endoscopy. All endoscopes used in the study were also used to examine patients attending the hospital, and 50 of each of the upper and lower gastrointestinal endoscopes were randomly selected and assigned to the CB and BB groups using the coin flip method. The primary outcomes were residual organic materials after the cleaning process, as determined by on-site test strip and adenosine triphosphate (ATP) tests. The secondary outcomes were the microorganism culture results.
The novel BB (EZ Jet Clean Ball; Silverex, Incheon, Korea) is made to fit the endoscopic channel, and balls of various sizes (2.2, 2.8, 3.2, 3.7, and 4.2 mm) are used according to the diameter of the lumen. It is made of a silicon ball wrapped in microfibers that are 0.5–1.0 mm larger than the inner diameter of the endoscopic channel. Upon contact with water, the ball swells slightly from its original size (approximately 0.1 mm) (Supplementary Fig. 1). It is subsequently sucked in using the tip of the endoscope. Its design allows it to scrub the interior as it passes through the lumen of the channel (Fig. 1). The upper gastrointestinal endoscopes used for the examination were GIF-Q260 and GIF-H260 (Olympus, Tokyo, Japan; channel diameter, 2.8 mm; universal code diameter, 3.7 mm). CF-Q260AL, CF-H260AL, and CF-HQ290 (Olympus) were used as the lower gastrointestinal endoscopes (channel diameter, 3.2, 3.7, and 3.7 mm, respectively; universal code diameter, 3.7 mm for all).
The endoscope reprocessing process was performed in accordance with the standard method commonly recommended by the American Society for Gastrointestinal Endoscopy,7 European Society of Gastrointestinal Endoscopy,5 and Korean Society of Gastrointestinal Endoscopy.12 Briefly, precleaning was performed at the point of use after the endoscopic examination, after which the endoscope was moved to the reprocessing area, and manual cleaning was performed. During the manual cleaning procedure in the BB group, two BBs of appropriate size were placed in the enzymatic detergent solution and aspirated up the channel from the distal tip. Subsequently, the suction valve was removed, and a BB of an appropriate size of the universal cord (3.7 mm) was installed in the suction cylinder. The suction button was reinstalled, and the solution was aspirated again to clean the universal cord. In the CB group, the sterilized disposable CB (VS-20B; Vision Medical, Incheon, Korea) method was used; the brush was inserted in the direction of the universal cord and endoscopic distal end.
Samples were taken immediately after channel-cleaning for both groups, and the flush-brush-flush method adapted from the Duodenoscope Surveillance Sampling and Culturing Protocols, developed jointly by the Department of Health and Human Services, Food and Drug Administration; Centers for Disease Control and Prevention; and American Society for Microbiology, was used.15 Briefly, a sample collection container was placed under the distal tip of the endoscope, and 20 mL of saline was injected into the biopsy port to flush the instrument channel and collect the fluid in the sample collection container. In addition, air was flushed into the instrument channel to collect residual fluid. A sterilized channel-cleaning brush was then inserted into the biopsy port to collect the fluid from the tip. The brush head that came out through the tip was also cut off with a wire cutter and collected in a container. Subsequently, the fluid flushing process with saline and air was repeated.
All tests, including microorganism culture, were performed with samples obtained immediately after manual cleaning (not after the completion of all reprocessing procedures). Immediately after fluid collection, the presence of residual carbohydrate, protein, and hemoglobin was assessed on-site using a ChannelCheck16 test strip (Healthmark Industries, Fraser, MI, USA) by the reprocessing personnel. This involved dipping the strip in the collected fluid for 5 seconds, waiting for 90 seconds, and observing the color change. The lower limits of detection for carbohydrate, protein, and hemoglobin were 25, 30, and 0.25 µg/mL, respectively. The microorganism burden was measured with the ATP level using a luminometer17 (Lumitester Smart; Kikkoman Corp., Chiba, Japan), which is a commercially available on-site ATP measuring kit that uses a swab (Lucipac A3;18 Kikkoman Corp.) immediately after fluid collection by the reprocessing personnel. The ATP levels were measured in relative light units (RLU). If any strip test was positive for any organic residues or the ATP test showed >40 RLU, the cleaning process was performed again and retested to confirm a negative strip test and ensure that the RLU value was lowered to <40.18
To check for the presence of microorganisms, 45 mL of Dey-Engley neutralizing broth (Sigma-Aldrich, St. Louis, MO, USA) was added to the fluid collected in the sample collection container and then vortexed for 10–20 seconds. The fluid was subsequently transferred to a conical tube and centrifuged at 3,500×g for 15 minutes. Then, the pellet was spread on a blood agar plate and incubated at 35°C–37°C for 72 hours. Microbial cultures were performed in Nowon Eulji Medical Center, Eulji University laboratory.
Since previous studies on the detection of residual organic material and microorganisms after the endoscope cleaning process could not be found, the target number of samples in each group was arbitrarily set to 50, with 100 samples. Continuous variables (ATP level) are presented as mean±standard deviation, and Student t-test was used to compare the two groups. Categorical variables (presence of organic material and microorganism culture) are presented as numbers (%) and analyzed using the chi-square test. The measured values are presented in terms of the frequency and fraction (%). Double-sided p-values of <0.05 were considered statistically significant. All statistical analyses were conducted using R software (R for Windows V.4.0.0; The R Foundation for Statistical Computing, Vienna, Austria).
The study protocol was reviewed and approved by the Institutional Review Board of Nowon Eulji Medical Center, Eulji University School of Medicine (IRB No: NON2020-002). As this was not a human study, informed consent was waived. The study was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki.
In total, 114 endoscopes were cleaned and tested, slightly exceeding the planned number. Twenty-nine upper and 27 lower endoscopes were cleaned with the CB, while 29 upper and 29 lower endoscopes were cleaned with the BB (Table 1). The endoscope models used for upper gastrointestinal endoscopy were GIF-Q260 (Olympus) and GIF-H260, which have a 2.8-mm-wide instrumental channel diameter. Furthermore, CF-Q260AL (instrumental channel diameter, 3.2 mm), CF-H260AL, and CF-HQ290 (instrumental channel diameter, 3.7 mm) were used for lower gastrointestinal endoscopy. There was no difference in the ratio of upper and lower gastrointestinal endoscopies (p=0.997) or proportion of the endoscope models (p=0.963) between the two groups. There were no cases in which the BB was stuck in the endoscopic channel.
After the cleaning process, both residual carbohydrates and proteins were detected in one (1.8%) and two endoscopes (3.4%) in the CB and BB groups, respectively (p=1.000). Similarly, residual proteins were also detected in one (1.8%) and two endoscopes (3.4%) in the CB and BB groups, respectively (p=1.000). Residual hemoglobin was positive in one (1.8%) and three endoscopes (5.2%) in the CB and BB groups (p=0.636) (Table 2). In the CB group, residual carbohydrate, protein, and hemoglobin were all detected in one endoscope, whereas in the BB group, residual carbohydrate, protein, and hemoglobin were all detected in two endoscopes, with only hemoglobin present in the other.
The ATP levels were 10.6±15.9 and 12.5±14.3 RLU in the CB and BB groups, respectively (p=0.496) (Fig. 2). In the BB group, two endoscopes measured the ATP levels of >40 RLU (44 and 100 RLU), and in the CB group, one endoscope measured the ATP levels of >40 RLU (117 RLU). Twenty-seven (48.2%) and 19 endoscopes (32.8%) in the CB and BB groups, respectively, were positive for microbial cultures (p=0.136). The ATP level and microbial culture results for each endoscope were categorized into the BB and CB groups and are presented in Figure 3 and Table 3.
Most microorganisms are removed during the cleaning and disinfection steps in endoscope reprocessing. Manual cleaning, including flushing and brushing of the entire channel system, is emphasized in most guidelines and is considered the most important step.5,7,12 Any debris that remains may impair the efficacy of subsequent reprocessing steps and support the formation of biofilms.10,11 When cleaning the endoscopic channel, a brush in the form of nylon bristles attached to a twisted stainless-steel wire is generally used. Outbreaks of carbapenemase-producing Klebsiella pneumoniae due to contamination of this brush have been reported,19 and the off-label use of the brush has been identified as a major cause.20 There is also a risk of damaging the lumen of the endoscopic channel by scrubbing with hard bristles and wire.5
The channel-cleaning BB used in this study has neither nylon bristles nor twisted stainless-steel wires. The BB is composed of microfibers that surround a soft silicon sphere. This allows it to pass through the channel while scrubbing it, thus possibly cleaning a wider cross-sectional area without causing damage and without the need for strenuous labor. Since cleaning the channel by suctioning the BB is a simple process of aspirating the BB along with the detergent solution, the entire process takes <1 minute and leaves little room for individual differences and can be easily standardized. It was feasible to use the BB to clean the endoscopic channel and quicker to use compared with the CB. Moreover, the BB was not stuck in the channel. It can be cumbersome to select and use a BB of an appropriate size, but it is not difficult once an individual becomes familiar with it because the channel size does not differ greatly for each endoscope despite the wide variety of endoscope models. When comparing the cleaning efficacy between the BB and CB, there was no significant difference in the presence of residual organic materials (protein, carbohydrate, and hemoglobin), ATP levels, and microbial cultures.
Indicators verifying the adequacy of the endoscope reprocessing process have not yet been clearly established. To date, various methods have been used or studied, such as a test strip for bioburden, measuring the ATP level, and a microorganism culture, as in the methods used in this study.16,21 However, it is also not clear how, when, and where the specimens should be collected as well as how the test results should be interpreted. In ATP tests, the cutoff values of <20022 or <100 RLU23 were suggested depending on the study. In our study, a more stringent cutoff value of ≤40 RLU was set by referring to the results of a previous study18 using the same equipment as in this study. Moreover, there was no significant difference in the mean ATP levels and the case of the ATP levels of >40 RLU between the two groups.
In this study, residual organic materials, although very few, were detected in both groups, and microbial culture tests were also positive in several endoscopes in both groups. In clinical practice, it is difficult to tolerate the presence of any residual organic materials or microbial cultures in the endoscope after reprocessing. However, this study was performed operated under a more stringent condition, as samples were acquired and tested immediately after manual cleaning and without performing other appropriate disinfection procedures. In addition, it is considered acceptable to have a positive microbial culture result without undergoing a high-level disinfection process. In the disinfection process, because the channel lumen is flushed and immersed using a high-level disinfectant, residual organic materials, and microorganisms are further reduced and expected to be eliminated after the completion of endoscope reprocessing.
Because of the narrow and long structure of the endoscopic channel, it is difficult to check it with the naked eye, as foreign substances are easily trapped, and defects are prone to occur during the cleaning process.24 In particular, when defoaming agents, lubricants, and tissue glue are used during endoscopy, these substances may not be well removed from the channel during endoscope reprocessing, which can cause problems.25 Therefore, various efforts have been made to clean the channel properly. Liu et al.26 attempted to use SpyGlass to inspect the lumen of the channel. Moreover, Thaker et al.13 suggested a method for inspecting the endoscope instrument channel using a prototype borescope. Meanwhile, to clean the inside of the channel, Bhatt et al.27 proposed a method of removing and sterilizing the biofilm inside the endoscopic channel using an argon plasma-activated gas with a special device.
In this study, the BB failed to show superiority to the CB in terms of efficacy. However, it is expected to increase the degree of compliance because of its simpler protocol and reduced labor requirements. In theory, as the cross-sectional area for scraping the channel lumen is wider than that of the CB, it may be expected that contaminants can be removed more accurately using a BB.
The limitation of this study is the fact that the number of samples was arbitrarily determined and statistically, the BB failed to show better results compared with the CB. Additionally, residual organic materials were detected at slightly higher levels in the BB group (three vs. one). The lack of significant differences between the groups may be attributable to the insufficient sample size. This requires further validation. However, the number of events was small, and clinically significant differences were not observed. Furthermore, the BB has several theoretical advantages, which need to be verified with more studies and in a clinical setting. In our study, only conventional upper and lower gastrointestinal endoscopes were used, and endoscopes with complex structures, such as duodenoscopes and linear echoendoscopes, were not used. In fact, the BB is used for channel-cleaning, and it is not considered superior to CB for cleaning complex structures such as the elevator mechanisms of these endoscopes. This finding may be further investigated in future studies.
In conclusion, the efficacy of BB was not significantly different from that of CB in the endoscopic channel-cleaning process.
Supplementary Material
Supplementary materials related to this article can be found online at https://doi.org/10.5946/ce.2021.210.
Notes
REFERENCES
1. Epstein L, Hunter JC, Arwady MA, et al. New Delhi metallo-β-lactamase-producing carbapenem-resistant Escherichia coli associated with exposure to duodenoscopes. JAMA. 2014; 312:1447–1455.
2. Kovaleva J, Peters FT, van der Mei HC, et al. Transmission of infection by flexible gastrointestinal endoscopy and bronchoscopy. Clin Microbiol Rev. 2013; 26:231–254.
3. Spach DH, Silverstein FE, Stamm WE. Transmission of infection by gastrointestinal endoscopy and bronchoscopy. Ann Intern Med. 1993; 118:117–128.
4. McCafferty CE, Aghajani MJ, Abi-Hanna D, et al. An update on gastrointestinal endoscopy-associated infections and their contributing factors. Ann Clin Microbiol Antimicrob. 2018; 17:36.
5. Beilenhoff U, Biering H, Blum R, et al. Reprocessing of flexible endoscopes and endoscopic accessories used in gastrointestinal endoscopy: Position Statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA): update 2018. Endoscopy. 2018; 50:1205–1234.
6. Son BK, Kim BW, Kim WH, et al. Korean Society of Gastrointestinal Endoscopy guidelines for endoscope reprocessing. Clin Endosc. 2017; 50:143–147.
7. Reprocessing Guideline Task Force, Petersen BT, Cohen J, et al. Multisociety guideline on reprocessing flexible GI endoscopes: 2016 update. Gastrointest Endosc. 2017; 85:282–294.e1.
8. Barakat MT, Girotra M, Huang RJ, et al. Scoping the scope: endoscopic evaluation of endoscope working channels with a new high-resolution inspection endoscope (with video). Gastrointest Endosc. 2018; 88:601–611.e1.
9. Barakat MT, Huang RJ, Banerjee S. Simethicone is retained in endoscopes despite reprocessing: impact of its use on working channel fluid retention and adenosine triphosphate bioluminescence values (with video). Gastrointest Endosc. 2019; 89:115–123.
10. Otter JA, Vickery K, Walker JT, et al. Surface-attached cells, biofilms and biocide susceptibility: implications for hospital cleaning and disinfection. J Hosp Infect. 2015; 89:16–27.
11. Ren-Pei W, Hui-Jun X, Ke Q, et al. Correlation between the growth of bacterial biofilm in flexible endoscopes and endoscope reprocessing methods. Am J Infect Control. 2014; 42:1203–1206.
12. Cheung DY, Jang BI, Kim SW, et al. Multidisciplinary and multisociety practice guideline on reprocessing flexible gastrointestinal endoscopes and endoscopic accessories. Clin Endosc. 2020; 53:276–285.
13. Thaker AM, Kim S, Sedarat A, et al. Inspection of endoscope instrument channels after reprocessing using a prototype borescope. Gastrointest Endosc. 2018; 88:612–619.
14. Buss AJ, Been MH, Borgers RP, et al. Endoscope disinfection and its pitfalls--requirement for retrograde surveillance cultures. Endoscopy. 2008; 40:327–332.
15. FDA/CDC/ASM Working Group on Duodenoscope Culturing. Duodenoscope surveillance sampling & culturing: reducing the risks of infection [Internet]. Atlanta (GA): Centers for Disease Control and Prevention;2018. [cited 2021 Mar 8]. Available from: https://www.cdc.gov/hai/organisms/cre/cre-duodenoscope-surveillance-protocol.html.
16. ASGE Technology Committee, Komanduri S, Abu Dayyeh BK, et al. Technologies for monitoring the quality of endoscope reprocessing. Gastrointest Endosc. 2014; 80:369–373.
17. Suzuki S, Nishimoto K, Igarashi T, et al. A novel bioluminescent cycling assay for ATP and AMP using pyruvate orthophosphate dikinase. In : Stanley PE, Kricka LJ, editors. Bioluminescence and chemiluminescence. Singapore: World Scientific;2002. p. 457–460.
18. Ridtitid W, Pakvisal P, Chatsuwan T, et al. Performance characteristics and optimal cut-off value of triple adenylate nucleotides test versus adenosine triphosphate test as point-of-care testing for predicting inadequacy of duodenoscope reprocessing. J Hosp Infect. 2020; 106:348–356.
19. Alrabaa SF, Nguyen P, Sanderson R, et al. Early identification and control of carbapenemase-producing Klebsiella pneumoniae, originating from contaminated endoscopic equipment. Am J Infect Control. 2013; 41:562–564.
20. Muscarella LF. Risk of transmission of carbapenem-resistant Enterobacteriaceae and related “superbugs” during gastrointestinal endoscopy. World J Gastrointest Endosc. 2014; 6:457–474.
21. Fushimi R, Takashina M, Yoshikawa H, et al. Comparison of adenosine triphosphate, microbiological load, and residual protein as indicators for assessing the cleanliness of flexible gastrointestinal endoscopes. Am J Infect Control. 2013; 41:161–164.
22. Alfa MJ, Fatima I, Olson N. Validation of adenosine triphosphate to audit manual cleaning of flexible endoscope channels. Am J Infect Control. 2013; 41:245–248.
23. Alfa MJ, Olson N. Simulated-use validation of a sponge ATP method for determining the adequacy of manual cleaning of endoscope channels. BMC Res Notes. 2016; 9:258.
24. Chu NS, McAlister D, Antonoplos PA. Natural bioburden levels detected on flexible gastrointestinal endoscopes after clinical use and manual cleaning. Gastrointest Endosc. 1998; 48:137–142.
25. Ofstead CL, Hopkins KM, Eiland JE, et al. Widespread clinical use of simethicone, insoluble lubricants, and tissue glue during endoscopy: a call to action for infection preventionists. Am J Infect Control. 2019; 47:666–670.
26. Liu TC, Peng CL, Wang HP, et al. SpyGlass application for duodenoscope working channel inspection: impact on the microbiological surveillance. World J Gastroenterol. 2020; 26:3767–3779.
27. Bhatt S, Mehta P, Chen C, et al. Efficacy of low-temperature plasma-activated gas disinfection against biofilm on contaminated GI endoscope channels. Gastrointest Endosc. 2019; 89:105–114.
Fig. 1.
Ball brush. (A) Ball brushes are composed of various sizes to fit the size of the endoscopic channel used and are 2.8, 3.2, and 3.7 mm in order from the left. It is made of a silicon ball wrapped in microfibers. (B) When the ball brush enters the water, it swells slightly from its original size. (C) When the ball brush is sucked in using the tip of the endoscope, it is designed to scrub the interior as it passes through the lumen of the endoscopic channel.
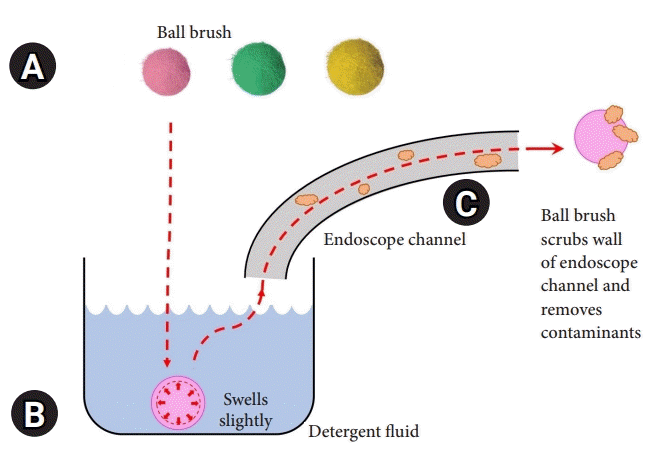
Fig. 2.
Comparison of the adenosine triphosphate (ATP) levels of the ball brush and conventional brush groups. The bar with whisker represents the mean and standard deviation. RLU, relative light unit.
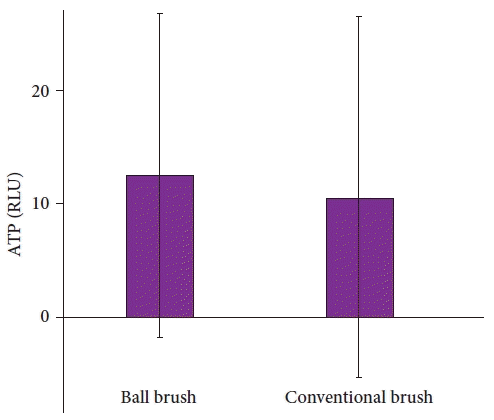
Fig. 3.
The adenosine triphosphate (ATP) level and microbial culture results for each endoscope. RLU, relative light unit.
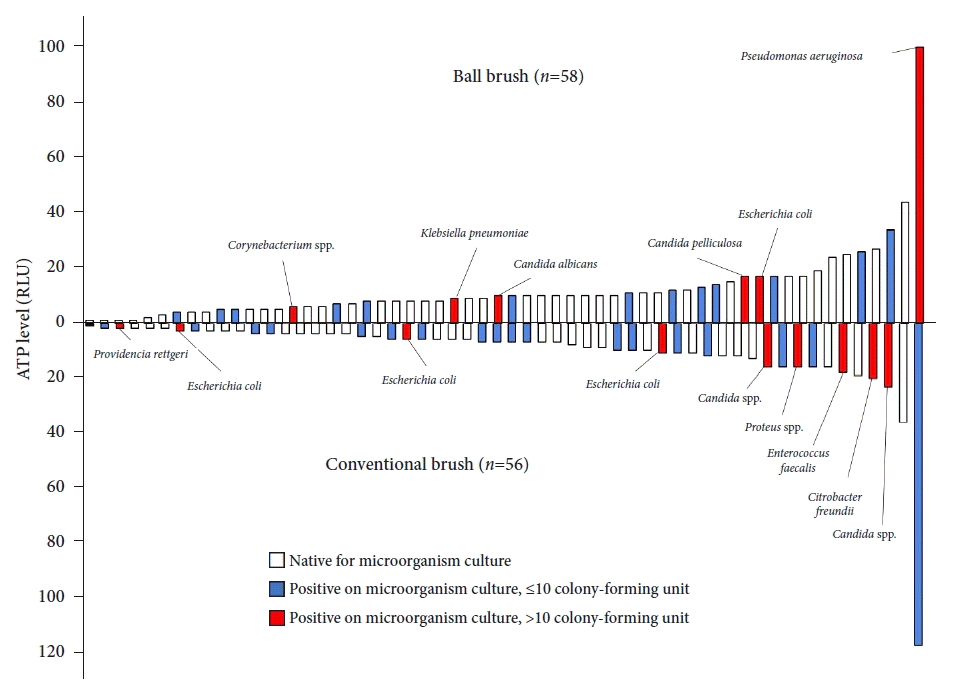
Table 1.
Details of endoscopes
Table 2.
Efficacy outcomes
Table 3.
Microbial culture results and corresponding ATP levels in both groups




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download