Abstract
Background
Urinary microRNA-21 (miR-21) has been reported to correlate with the histologic lesions of IgA nephropathy (IgAN). We investigated whether urinary or circulating miR-21 could serve as a biomarker for detecting the renal progression of IgAN.
Methods
Forty patients with biopsy-proven IgAN were enrolled in this study. Serum and urinary sediment miRs were extracted, and the expression of miR-21 was quantified by real-time quantitative polymerase chain reaction. Renal progression was defined as end-stage renal disease, a sustained doubling of serum creatinine, or a 50% decrease in estimated glomerular filtration rate (eGFR) from baseline.
Results
Six patients experienced renal progression during the follow-up period. The baseline eGFR was lower in the progression group (49±11 mL/min/1.73 m2 vs. 90±23 mL/min/1.73 m2, p<0.05) than in the non-progression group. The level of circulating miR-21 on kidney biopsy was higher in the progression group than in the non-progression group (40.0±0.6 vs. 38.2±1.1 ΔCt value of miR-21, p<0.01), whereas there was no significant difference in urinary miR-21 (38.1±2.1 vs. 37.8±1.4 ΔCt value of miR-21, p=0.687) between the two groups. Receiver operating characteristic curve analysis demonstrated that circulating miR-21 had good discriminative power for diagnosing renal progression of IgAN, with an area under the curve of 0.975.
IgA nephropathy (IgAN) is a mesangial proliferative glomerulonephritis with a variable clinical course ranging from asymptomatic urinary abnormalities to rapidly progressive kidney failure [1]. A key issue in the field is prediction of patient risk of rapid renal progression. The clinical predictors of renal outcome in IgAN, such as reduced renal function, proteinuria, and blood pressure during diagnosis, are acknowledged [2]. The histologic finding of IgAN is associated with development of end-stage renal disease [3]. However, recurrent renal biopsy is not an appropriate approach for evaluation of disease severity due to its invasive nature. Therefore, novel biomarkers of IgAN, which can differentiate individuals with disease from healthy people prior to renal biopsy, are needed to evaluate and administer timely and relevant management.
MicroRNAs (miRs) are non-coding, single-stranded RNA molecules that regulate pathological and physiological processes by a posttranscriptional mechanism [4]. Many miRs reveal organ-specific patterns of expression, with dysregulation being linked with diverse diseases [5]. Several studies have reported the relationship between miRs and glomerulonephritis [6-8]. Among all miRs, miR-21 has been researched extensively in nephrology including glomerulonephritis [9-11]. In IgAN, intra-renal expression of miR-21 was reported to reflect renal fibrosis and renal survival [7]. However, there are few studies that investigate the role of circulating miR-21 in progression of IgAN. Therefore, we performed this study to determine if a circulating miR-21 could serve as a marker of progression of IgAN.
Ethical statements: This study was approved by the Institutional Review Board of the Presbyterian Medical Center, Jeonju, South Korea (approval number: 2014-07-032). Written informed consent from participants was obtained prior to sample collection. This research abided by the principles of the Declaration of Helsinki.
From 2014 to 2020, a total of 40 patients with biopsy-proven IgAN were enrolled in this study. The inclusion criteria were patients ≥18 years who had provided informed consent. The exclusion criteria are as follows: patients with secondary IgAN; patients who had received immunosuppressants including corticosteroids before enrollment of this study, patients with systemic diseases such as diabetes, patients who underwent renal replacement therapy including renal transplantation or dialysis, pregnant patients, and ≤8 glomeruli in renal biopsy samples. Following renal biopsy, the patients received angiotensin-converting enzyme inhibitors/angiotensin receptor blockers or corticosteroids. In this study, the enrolled patients were followed for more than 1 year.
All data including clinical history were obtained through retrospective chart review during renal biopsy. The clinical end-point was defined as a composite of any of the following events over the study duration: 50% decline of estimated glomerular filtration rate (eGFR) from baseline, persistent doubling of serum creatinine, or initiation of renal replacement therapy. Patients who developed the clinical end-point during follow-up were included in the progression group. The eGFR was evaluated using the abbreviated Modification of Diet in Renal Disease equation [12]. A pathologist diagnosed with IgAN based on three microscopic analyses of the specimens and categorized the observed lesion into one of five classes consistent with the Oxford classification [13]. The degree of mesangial proliferation, glomerular sclerosis, inflammation, and interstitial fibrosis, as well as tubular atrophy were assessed from the Masson’s trichrome-stained sections of each biopsy utilizing a semi quantitative method. The percentage of cortical area involved in interstitial fibrosis or tubular atrophy was quantitated. A score of T0, T1, or T2 was conferred if the percentage of involved cortical area was 0%–25%, 26%–50%, or >50%, respectively. Herein, T0-T1 and T2 findings were defined as mild and severe renal fibrosis, respectively.
Total RNA was isolated from urinary exosomes employing the mirVana PARIS total RNA isolation kit (Life Technologies, Carlsbad, CA, USA; Cat. #AM1556) as per the manufacturer’s protocol. For the endogenous small RNA control, we incorporated cel-mir-39 (25 fmol, Life Technologies Cat. #4464066) to each sample, as previously described [14]. Using the TaqMan MicroRNA reverse transcription kit (Life Technologies, Cat. #4366596), a fixed RNA content of 4.8 ng from RNA elute was reverse transcribed. For quantitative real-time polymerase chain reactions (qRT-PCRs), 1.33 μL of the reverse transcription product was incorporated with 10 μL of TaqMan universal master mix (Cat. #4440038), 7.67 μL of H2O, and 1 μL of primers including miR-21 (Life Technologies, Cat. #4440887, assay ID:000397) in a 20 μL final reaction volume. The qRT-PCR was conducted on an Applied Biosystems (Waltham, MA, USA) 7500 Real-Time PCR system at 50°C for 2 minutes, 95°C for 10 minutes, and 40 cycles of 95°C for 15 seconds and 60°C for 1 minute [15,16]. The values of the threshold cycle were computed using SDS 1.4.1 software (Applied Biosystems). All qRT-PCR reactions were performed in triplicate. The average expression levels of miR-21 were normalized utilizing cel-mir-39 (Applied Biosystems) and subsequently analyzed by the two (median cel-mir-39 Ct value–average Ct value of the given sample) method, as previously described [16-18]. All data were visualized using GraphPad Prism version 5 (GraphPad Software, San Diego, CA, USA). p-values <0.05 were considered statistically significant.
All data are presented as mean±standard deviation unless otherwise specified. The baseline characteristics of patients in the non-progression and progression groups were compared using the chi-square test, t-tests, or Fisher exact test, as appropriate. Clinically, the relevant that were significantly associated with renal progression on univariate analysis were subjected to multivariate analysis using binary logistic regression. Statistical significance was noted at a p-value <0.05. All statistical analyses were performed using the SPSS software, version 22.0 (IBM Corp., Armonk, NY, USA).
Among 40 patients with IgAN, the mean age was 39±14 years, 40% were male, and the mean follow-up duration was 43 months (range, 6–84 months). The value of mean eGFR and proteinuria were 85±25 mL/min/1.73 m2 and 1,584±2,338 mg/dL, respectively. There were no histologic differences except baseline renal function and fibrosis. In the Oxford classification, patients with progression had remarkably higher T2 scores than those without progression. Patients with progression had a poorer renal function (49±11 mL/min/1.73 m2 vs. 90±23 mL/min/1.73 m2, p<0.01) on admission and more frequent severe renal fibrosis (50% vs. 0%, p=0.002) than that observed in the non-progression group. The level of circulating miR-21 (40.0±0.6 vs. 38.2±1.1 miR-21 ΔCt value, p<0.01) was higher in the progression group than in the non-progression group, while the level of urinary miR-21 did not differ between the two groups (38.1±2.1 vs. 37.8±1.4 miR-21 ΔCt value, p=0.687) (Table 1).
Circulating miR-21 levels were directly correlated with proteinuria (Pearson’s correlation=0.337, p=0.033). However, we detected a significant inverse correlation between circulating miR-21 level and eGFR (Pearson’s correlation=–0.330, p=0.037) (Fig. 1). The area under the receiver operator characteristic (ROC) curve was 0.975 for circulating miR-21 (Fig. 2). Additionally, the level of circulating miR-21 was higher (40.2±0.6 vs. 38.3±1.2 ΔCt value of miR-21, p=0.013) in patients with severe renal fibrosis (Fig. 3).
Herein, we revealed that circulating miR-21 level was higher in patients with IgAN with renal progression than in those without renal progression and correlated directly with proteinuria on kidney biopsy. Additionally, the ROC curve analysis for circulating miR-21 depicted good discriminative ability for predicting IgAN renal progression. Therefore, circulating miR-21 could be a surrogate marker for renal progression of IgAN. Our results offer a rationale for employing circulating miR-21 as a biomarker for prediction of clinical outcome of IgAN.
The clinical course of IgAN is extremely diverse [1]. Therefore, the examination of non-invasive and more reliable biomarkers to evaluate disease progression is imperative for a clinician. Recently, miR has been in the spotlight as a disease biomarker since multiple miRs exhibit differential expression in diverse organs [5]. Previous studies demonstrated that miR-21 plays a central role in inflammation, stress response, and apoptosis [15,19]. Up-regulation of miR-21 expression has been reported to be correlated with several renal disorders including diabetic nephropathy and acute kidney injury [20,21]. Liang et al. [7] reported that the level of miR-21 in urinary sediment and intra-renal expression of miR-21 were related to histologic findings of IgAN. In our study, the urinary miR-21 level was higher in the progression group than that in the non-progression group. However, the difference was not statistically significant. We believe that this might be due to the small sample size in this study.
Previous miR studies in renal disorders including glomerulonephritis have focused on urinary miRs [5]. Urinary levels of miR-146a and miR-155 are increased in patients with IgAN and associated with proteinuria. Additionally, urinary miR-30d and miR-10a levels in focal segmental glomerulosclerosis (FSGS) are high in mouse and human and may signify a novel biomarker of kidney injury [22]. Recently, understanding of pathological or physiological roles was increased by the discovery of circulating miRs, although their exact role is obscure. Thus, circulating miRs are regarded as fascinating biomarker candidates and may reflect kidney disease [17]. Differentially expressed circulating miRs have been discovered in patients with diverse glomerular diseases including IgAN [23]. A previous study explored the specific profile of circulating miRs of nephrotic syndrome such as FSGS, proposing a possibility of biomarker [24]. In addition, Sun et al. [25] recently reported that circulating miRs in extracellular vesicles can be beneficial in identifying idiopathic membranous nephropathy among nephrotic syndrome and predicting the treatment response in patients.
Some researchers also imply the roles of diagnostic, prognostic, or therapeutic biomarker for miRs in patients with IgAN [26,27]. Hu et al. [26] demonstrated that plasma miR-29a could be a marker for reflecting renal damage and predicting IgAN progression. Li et al. [27] suggested that miR-23b may be an interesting future therapeutic target for treatment of IgAN. Interestingly, in this study, the level of circulating miR-21 was higher in the progression group than in the non-progression group. Furthermore, those levels correlated directly with proteinuria and inversely with eGFR during kidney biopsy, which was already known as an IgAN predictor [2]. The level of circulating miR-21 was also associated with renal fibrosis in this study. Therefore, we propose that circulating miR-21 could be useful to predict IgAN progression. However, the level of urinary miR-21 was not related to clinical parameters. Therefore, to illuminate the role of circulating and urinary miR-21 in IgAN; to confirm our results, larger, prospective, randomized, and controlled trials are required.
Apart from miR-21, several small studies have reported miR expression in IgAN as a diagnostic or prognostic biomarker [6-8,22,28-31]. Serino et al. [30] showed that a miR-148b was involved in the pathogenesis of IgAN, explaining the aberrant glycosylation of IgA1. Subsequently, circulating miR-148b and miR-let-7b were demonstrated to be helpful in discriminating patients with IgAN from healthy controls and patients with other types of primary glomerulonephritis [31]. However, there are some questions when miRs are used as biomarkers for renal diseases including IgAN. Since most miRs target multiple proteins, one miR can be associated with diverse diseases, reducing the unique role in differentiation from pathology and physiology [5]. Consequently, finding causes for changes in this level is challenging. Another hurdle to overcome for being a biomarker in renal disorders is the method of identification of miRs. To inspect circulating miRs, several approaches have emerged including qRT-PCR, microarrays, and next-generation sequencing. Every method has its pros and cons such as quantification, simplicity, and validity [32]. The sensitivity and specificity of these techniques are often dependent on the sample type and volumes of plasma or serum. Furthermore, the methods are not standardized or validated among research laboratories [32]. Therefore, discreet validation and standardization are required before the miRs are translated to clinical decision-making. Of these, qRT-PCR is the most advantageous for analyzing diverse specific miR due to its simplicity and speed. However, controversy on selection of the most appropriate endogenous reference genes for circulating miRs expression level normalization has been ongoing [33]. Therefore, to broaden the clinical utility for miRs, such methodologic issues should be resolved.
Our study has some limitations. First, this was a single-center study with a small number of participants. Therefore, larger, prospective, randomized, and controlled trials are needed. Second, we did not enroll a healthy control in the present study. Third, we evaluated the urinary miR expression in urinary sediments, which are composed of varying cell types, but did not evaluate their cellular source. Finally, we did not evaluate the miR-21 expression in kidney biopsy specimen.
In conclusion, we found increased circulating miR-21 level in IgAN patient with renal progression compared to that in patients without renal progression. Furthermore, circulating miR-21 level on kidney biopsy was associated with proteinuria and baseline renal function. Therefore, circulating miR-21 could be utilized as a biomarker for IgAN prognosis. To confirm our results, larger, prospective, randomized, and controlled studies are required in the future.
Notes
Conflicts of interest
This research was supported by Daewon Pharmaceutical Company. Except for that, no potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: AYC, IOS. Data curation: JHO, KYL. Formal analysis: AYC, IOS. Funding acquisition: IOS. Investigation: IOS. Methodology: AYC, IOS. Project administration: AYC, IOS. Resources: IOS. Software: IOS. Supervision: IOS. Validation: IOS. Visualization: AYC, IOS. Writing - original draft: AYC, IOS. Writing - review & editing: AYC, IOS. Approval of final manuscript: all authors.
References
1. Moriyama T, Tanaka K, Iwasaki C, Oshima Y, Ochi A, Kataoka H, et al. Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PLoS One. 2014; 9:e91756.
2. Le W, Liang S, Hu Y, Deng K, Bao H, Zeng C, et al. Long-term renal survival and related risk factors in patients with IgA nephropathy: results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant. 2012; 27:1479–85.
3. Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009; 76:546–56.
4. Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008; 9:102–14.
5. Sun IO, Lerman LO. Urinary microRNA in kidney disease: utility and roles. Am J Physiol Renal Physiol. 2019; 316:F785–93.
6. Xu BY, Meng SJ, Shi SF, Liu LJ, Lv JC, Zhu L, et al. MicroRNA-21-5p participates in IgA nephropathy by driving T helper cell polarization. J Nephrol. 2020; 33:551–60.
7. Liang S, Cai GY, Duan ZY, Liu SW, Wu J, Lv Y, et al. Urinary sediment miRNAs reflect tubulointerstitial damage and therapeutic response in IgA nephropathy. BMC Nephrol. 2017; 18:63.
8. Szeto CC, Wang G, Ng JK, Kwan BC, Mac-Moune Lai F, Chow KM, et al. Urinary miRNA profile for the diagnosis of IgA nephropathy. BMC Nephrol. 2019; 20:77.
9. Chung AC, Dong Y, Yang W, Zhong X, Li R, Lan HY. Smad7 suppresses renal fibrosis via altering expression of TGF-β/Smad3-regulated microRNAs. Mol Ther. 2013; 21:388–98.
10. Lan HY, Chung AC. TGF-β/Smad signaling in kidney disease. Semin Nephrol. 2012; 32:236–43.
11. Trionfini P, Benigni A, Remuzzi G. MicroRNAs in kidney physiology and disease. Nat Rev Nephrol. 2015; 11:23–33.
12. Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007; 53:766–72.
13. Trimarchi H, Barratt J, Cattran DC, Cook HT, Coppo R, Haas M, et al. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017; 91:1014–21.
14. Park MY, Herrmann SM, Saad A, Widmer RJ, Tang H, Zhu XY, et al. Circulating and renal vein levels of microRNAs in patients with renal artery stenosis. Nephrol Dial Transplant. 2015; 30:480–90.
15. Xu X, Kriegel AJ, Jiao X, Liu H, Bai X, Olson J, et al. miR-21 in ischemia/reperfusion injury: a double-edged sword? Physiol Genomics. 2014; 46:789–97.
16. Sole C, Moline T, Vidal M, Ordi-Ros J, Cortes-Hernandez J. An exosomal urinary miRNA signature for early diagnosis of renal fibrosis in lupus nephritis. Cells. 2019; 8:773.
17. Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008; 105:10513–8.
18. Lopes CB, Magalhaes LL, Teofilo CR, Alves APNN, Montenegro RC, Negrini M, et al. Differential expression of hsa-miR-221, hsa-miR-21, hsa-miR-135b, and hsa-miR-29c suggests a field effect in oral cancer. BMC Cancer. 2018; 18:721.
19. Zhou J, Wang KC, Wu W, Subramaniam S, Shyy JY, Chiu JJ, et al. MicroRNA-21 targets peroxisome proliferators-activated receptor-alpha in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci U S A. 2011; 108:10355–60.
20. Fouad M, Salem I, Elhefnawy K, Raafat N, Faisal A. MicroRNA-21 as an early marker of nephropathy in patients with type 1 diabetes. Indian J Nephrol. 2020; 30:21–5.
21. Yun CY, Lim JH, Oh JH, Cho AY, Lee KY, Sun IO. Urinary exosomal microRNA-21 as a marker for scrub typhus-associated acute kidney injury. Genet Test Mol Biomarkers. 2021; 25:140–4.
22. Wang N, Zhou Y, Jiang L, Li D, Yang J, Zhang CY, et al. Urinary microRNA-10a and microRNA-30d serve as novel, sensitive and specific biomarkers for kidney injury. PLoS One. 2012; 7:e51140.
23. Wang Z, Liao Y, Wang L, Lin Y, Ye Z, Zeng X, et al. Small RNA deep sequencing reveals novel miRNAs in peripheral blood mononuclear cells from patients with IgA nephropathy. Mol Med Rep. 2020; 22:3378–86.
24. Ramezani A, Devaney JM, Cohen S, Wing MR, Scott R, Knoblach S, et al. Circulating and urinary microRNA profile in focal segmental glomerulosclerosis: a pilot study. Eur J Clin Invest. 2015; 45:394–404.
25. Sun IO, Bae YU, Lee H, Kim H, Jeon JS, Noh H, et al. Circulating miRNAs in extracellular vesicles related to treatment response in patients with idiopathic membranous nephropathy. J Transl Med. 2022; 20:224.
26. Hu H, Wan Q, Li T, Qi D, Dong X, Xu Y, et al. Circulating MiR-29a, possible use as a biomarker for monitoring IgA nephropathy. Iran J Kidney Dis. 2020; 14:107–18.
27. Li H, Chen Z, Chen W, Li J, Liu Y, Ma H, et al. MicroRNA-23b-3p deletion induces an IgA nephropathy-like disease associated with dysregulated mucosal IgA synthesis. J Am Soc Nephrol. 2021; 32:2561–78.
28. Tan K, Chen J, Li W, Chen Y, Sui W, Zhang Y, et al. Genome-wide analysis of microRNAs expression profiling in patients with primary IgA nephropathy. Genome. 2013; 56:161–9.
29. Pawluczyk IZA, Didangelos A, Barbour SJ, Er L, Becker JU, Martin R, et al. Differential expression of microRNA miR-150-5p in IgA nephropathy as a potential mediator and marker of disease progression. Kidney Int. 2021; 99:1127–39.
30. Serino G, Sallustio F, Cox SN, Pesce F, Schena FP. Abnormal miR-148b expression promotes aberrant glycosylation of IgA1 in IgA nephropathy. J Am Soc Nephrol. 2012; 23:814–24.
31. Serino G, Pesce F, Sallustio F, De Palma G, Cox SN, Curci C, et al. In a retrospective international study, circulating miR-148b and let-7b were found to be serum markers for detecting primary IgA nephropathy. Kidney Int. 2016; 89:683–92.
32. Ono S, Lam S, Nagahara M, Hoon DS. Circulating microRNA biomarkers as liquid biopsy for cancer patients: pros and cons of current assays. J Clin Med. 2015; 4:1890–907.
33. Roberts TC, Coenen-Stass AM, Wood MJ. Assessment of RT-qPCR normalization strategies for accurate quantification of extracellular microRNAs in murine serum. PLoS One. 2014; 9:e89237.
Fig. 1.
Correlation of circulating microRNA-21 (miR-21) with clinical parameters. Circulating miR-21 levels correlated directly with proteinuria (A) and inversely with the estimated glomerular filtration rate (eGFR) (B).
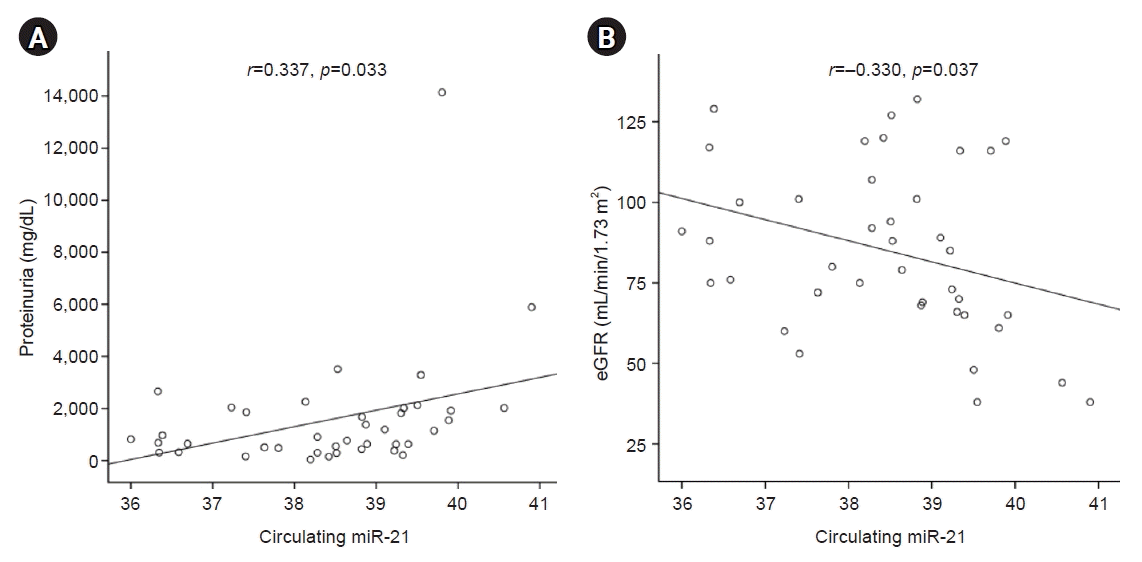
Fig. 2.
Receiver operating characteristic curve and performance for circulating microRNA-21 (miR-21) at the time of kidney biopsy. The area under the receiver operating characteristic curve (AUC) was 0.975 for circulating miRNA-21.
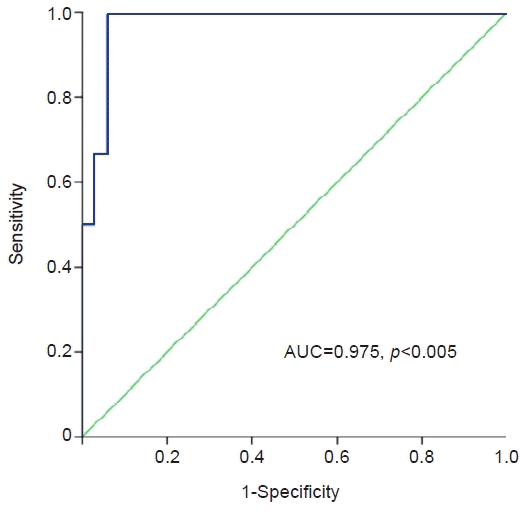
Fig. 3.
Circulating microRNA-21 (miR-21) levels were higher in patients with severe fibrosis than in those with mild fibrosis. After reference gene normalization, the relative expression intensity of circulating miR-21 was higher in patients with severe fibrosis than in patients with mild fibrosis.
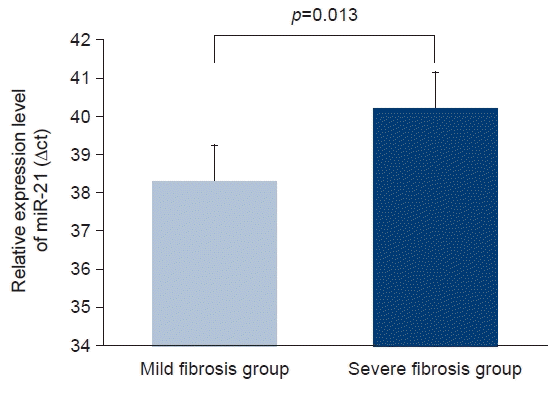
Table 1.
Comparison of baseline characteristics according to whether patients experienced progression




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download