Abstract
Purpose
Unfractionated heparin (UFH) is more commonly used as an anticoagulant after digital replantation than low-molecular-weight heparin (LMWH). We compared the success and complication rates of these two anticoagulants, since only a few studies have made this comparison directly.
Methods
Forty-four patients who underwent digital replantation for complete or incomplete digital amputation in the past 7 years at a single institution were included. The patients were divided into LMWH and UFH groups according to the anticoagulant administered. The success rates for each group were obtained, and the postoperative serum aspartate aminotransferase (AST) and alanine transaminase (ALT) levels were analyzed to compare the complication rates.
Results
All patients, except one, had successful recovery of circulation after replantation, and the success rate did not show a statistically significant difference between the two groups. The statistical analysis showed that the proportion of patients with abnormal serum AST or ALT levels in the LMWH group was significantly lower than that in the UFH group.
Conclusion
Although there was no significant difference in the success rate between the two groups, the risk of hepatotoxicity was significantly lower in the LMWH group than in the UFH group. Considering the advantages of LMWH, its extensive use is highly recommended for anticoagulation therapy in patients after digital replantation.
Heparin and its derivatives have been widely used in anticoagulation therapy after digital replantation to reduce thrombotic events and ensure robust blood flow [1]. There is no consensus among microsurgery centers due to lack of standardized protocols. The protocols used are mainly based on subjective clinical experience rather than evidence-based guidelines. Some institutions use unfractionated heparin (UFH) as the main anticoagulant after free tissue transfer, whereas others use low-molecular-weight heparin (LMWH), acetylsalicylic acid, thrombolytics, dextran, or prostaglandin E1 [1-6]. We only found one relevant systematic review in the Cochrane database. However, few studies have reviewed and compared the success rates of LMWH vs. UFH. Nevertheless, the methodological and statistical limitations of these studies have led to the lack of conclusions [7]. Moreover, the complication rates associated with LMWH or UFH were unclear, making it difficult for microsurgeons to choose between them [7]. In other words, the protocols are reliant on individual clinical experience, which can be biased.
We focused on hepatotoxicity among the complication of anticoagulant use. In our institution, we routinely perform liver function or anticoagulation panel tests perioperatively to monitor hepatotoxicity based on our clinical experience. Although well described in the literature, hepatotoxicity is often overlooked due to its asymptomatic nature, with a rare possibility of sequelae; however, aggravated symptoms can sometimes occur [2,8-13]. The rates of hepatotoxicity vary among studies, with most authors reporting higher rates with LMWH. However, this finding has not been statistically proven [10,14,15].
In previous studies, the comparison of hepatotoxicity rates between LMWH and UFH has been limited to particular instances, such as prophylaxis or treatment of pulmonary embolism. However, a comparison of hepatotoxicity rates between perioperative LMWH and UFH administration after digital replantation has not been presented. By comparing the hepatotoxicity, success rate, and complication rate between patients who received LMWH or UFH, the study aimed to promote the usage of LMWH over UFH as the main anticoagulant.
Ethics statement: This study was approved by the Institutional Review Board of Korea University Ansan Hospital (No. 2022AS1097) with a waiver of informed consent due to its retrospective nature. Written informed consent was obtained for publishing accompanying images of this report.
The medical records of patients who received digital replantation and perioperative anticoagulation therapy after complete digital amputation from 2014 to 2020 were reviewed. All patients underwent surgery by a single senior surgeon.
Patients were divided into two groups; LMWH and UFH. The demographics, presentation, treatment, success or failure of replantation and any major or minor complications were compared between the two groups. The investigated demographic variables included age, smoking history, preexisting medical conditions, zone of injury, elapsed time before surgery, and operation time.
All patients were administered either UFH or LMWH. UFH (Choongwae Heparin Sodium Inj., JW Pharmaceutical, Seoul, Korea) was administered intravenously. The patients were administered 2,500 IU/day for a week, and the drug dose was tapered to 500 IU/day. In the LMWH group, 20 mg of LMWH (Clexane Inj., Sanofi-Aventis Korea, Seoul, Korea) was subcutaneously injected once a day for 5 days. Anticoagulants were not discontinued unless the patients showed signs of associated complications. Despite an increase in liver function parameters, anticoagulation therapy was continued unless the patients showed marked symptoms, which did not occur in any of the included patients.
We defined failure (marked as “failure”) as per the following three categories described in a previous study [7]: (1) requiring surgical re-exploration, (2) requiring incision, or (3) microvascular insufficiency due to arterial and venous occlusions. We further defined successful replantation (marked as “success”) as the condition that was not marked as a failure.
All possible complications were identified, including hematoma and major or minor systemic bleeding, which are common side effects of anticoagulants [16]. We paid particular attention to hepatotoxicity as no severe or systemic complications occurred in the patients included in this study. The liver function test panel was thoroughly analyzed and compared between the two groups.
When the peak levels of serum aspartate aminotransferase (AST) or alanine transaminase (ALT) were above three times the upper normal limit (UNL), the condition was marked as “hepatotoxicity,” as described in a previous study [10]. The UNLs of AST and ALT were 40 and 41 U/L, respectively. Any value below three times the UNL was considered insignificant and labeled as “normal.” Any abnormalities in liver function tests, other than serum AST or ALT levels, were also investigated.
Basic demographic data were compared using the independent t-test (for continuous variables) and Fisher exact test (for categorical variables). The success and complication rates were compared using Fisher exact test. Statistical analyses were performed using IBM SPSS Statistics ver. 22.0 (IBM Corp., Armonk, NY, USA).
A total of 44 patients underwent digital replantation and perioperative anticoagulation therapy. Ten patients were classified into LMWH group and 34 patients into UFH group. The patient demographics are summarized in Table 1. Representative cases are shown in Figs. 1 and 2. The median patient age was 44.0±14.8 years. Among the patients, 59.1% were non-smokers and 40.9% were smokers. Regarding medical history, none of the patients had a history of hepatic diseases, and most patients had no comorbidities (59.1%). Most patients underwent replantation of one digit (72.7%), whereas a small number of patients underwent operations for more than one digit (27.2%). The success rate was 100% in the LMWH group, whereas one replantation failed in the UFH group (2.9%; category 3, microvascular insufficiency due to arterial and venous occlusions). There was no statistically significant difference in the success rates between the two groups (p>0.999) (Table 2, Fig. 3). No major complications, including systemic bleeding or thrombocytopenia, were observed in either group. The rate of hepatotoxicity was higher in the UFH group than in the LMWH group. The proportion of patients with elevated AST levels (50.0% and 10.0%, respectively; p=0.031) and the rate of ALT elevation (47.1% and 10.0%, respectively; p=0.027) were higher in the UFH group than in the LMWH group (Table 3, Fig. 4).
LMWH is a relatively new class of anticoagulants compared to UFH and is derived from UFH. The anticoagulant activity of LMWH originates from the inhibition of activated coagulation factor X (factor Xa), whereas the anticoagulant activity of UFH originates from its binding to the antithrombin III molecule [17,18]. Since its introduction in the 1970s, LMWH has gradually replaced UFH in clinical practice and has been used to prevent deep venous thrombosis, pulmonary embolism and to treat unstable angina and ST-elevation myocardial infarction [17,18].
However, many institutions still use UFH as the main anticoagulant therapy for perioperative anticoagulation in the microsurgical domain. Recently, there have been changes based on advances in the knowledge of flap dynamics and LMWH [1]. However, it is interesting to consider the advantages of LMWH compared to those of UFH. Some of the advantages of LMWH include: (1) improved predictability of dose-response relationships due to the lower rate of binding to plasma proteins [19], (2) increased plasma half-life due to a lower rate of binding to macrophages and endothelial cells, and (3) lower incidence of heparin-induced thrombocytopenia [20].
The hepatotoxicity of heparin and heparin-derived products has been described in several studies. However, heparin-related hepatotoxicity is often neglected due to its asymptomatic nature. Although heparin-related hepatotoxicity does not cause any sequelae in most cases, it should be closely monitored since it can result in elevated levels of liver enzymes and sometimes shows aggravated symptoms [11].
Our results showed that although patients who were administered UFH had a higher rate of hepatotoxicity than those who were administered LMWH, the success rate was not different. This finding is in agreement with a previous study [7], which showed no statistically significant differences between the two groups in terms of patient demographics. This could indicate that the hepatotoxicity of LMWH described in the literature could be exaggerated. There is no evidence to suggest that LMWH is associated with a high risk of hepatotoxicity in clinical settings. The results section does not include details of other liver function test panel results, such as bilirubin or ALT levels, since these were normal. Although there has been a recent case report regarding the elevation of bilirubin and ALT levels, neither is usually considered to be related to heparin-associated or enoxaparin-related hepatotoxicity [9].
The risk of hepatotoxicity due to UFH and LMWH was high in our results compared to that in other reports, which reported hepatotoxicity risks of 8% and 4%–13%, respectively [14,21]. This could be due to the small sample size or the underreported nature of heparin-derivative-induced hepatotoxicity. The mechanism underlying the different results is unknown, and further research is necessary.
Only enoxaparin was used in this study, despite the availability of several classes of LMWH in the market. This helped to reduce the burden of creating subgroups. Other classes of LMWH might have introduced additional variables, contributing to different results. Further, multicenter and double-blind clinical trials are needed to confirm our results.
A limitation of this study is the small sample size, and missing medical records owing to the retrospective nature of the study making the study underpowered. Moreover, the number of patients in the LMWH group was smaller than that in the UFH group. The use of LMWH in anticoagulant therapy in the perioperative period of digital replantation has only occurred recently at our institution. The indications for the use of LMWH are gradually expanding in almost all patients who undergo microsurgery, including digital replantation and tissue transfer. More detailed results, including a larger number of patients, will be presented in a subsequent prospective study.
References
1. Kaciulyte J, Losco L, Maruccia M, et al. Postsurgical antithrombotic therapy in microsurgery: our protocol and literature review. Eur Rev Med Pharmacol Sci. 2019; 23:4448–57.
2. Jokuszies A, Herold C, Niederbichler AD, Vogt PM. Anticoagulative strategies in reconstructive surgery: clinical significance and applicability. Ger Med Sci. 2012; 10:Doc01.
3. Maeda M, Fukui A, Tamai S, Mizumoto S, Inada Y. Continuous local intra-arterial infusion of antithrombotic agents for replantation (comparison with intravenous infusion). Br J Plast Surg. 1991; 44:520–5.
4. Pomerance J, Truppa K, Bilos ZJ, Vender MI, Ruder JR, Sagerman SD. Replantation and revascularization of the digits in a community microsurgical practice. J Reconstr Microsurg. 1997; 13:163–70.
5. Rodríguez Vegas JM, Ruiz Alonso ME, Terán Saavedra PP. PGE-1 in replantation and free tissue transfer: early preliminary experience. Microsurgery. 2007; 27:395–7.
6. Vretos KA, Tsavissis AG. Antithrombotic and antinflammatory drugs for protection of microvascular anastomosis. Acta Orthop Scand Suppl. 1995; 264:48–9.
7. Lin PT, Wang SH, Chi CC. Low molecular weight heparin for prevention of microvascular occlusion in digital replantation. Cochrane Database Syst Rev. 2020; 4:CD009894.
8. Baker EL, Loewenthal T, Salerno E, Baker WL. Probable enoxaparin-induced hepatotoxicity. Am J Health Syst Pharm. 2009; 66:638–41.
9. Bosco M, Kish T. Hepatotoxicity with elevated bilirubin secondary to prophylactic doses of unfractionated heparin: a case report and review of heparin-induced hepatotoxicity. J Pharm Technol. 2019; 35:36–40.
10. Carlson MK, Gleason PP, Sen S. Elevation of hepatic transaminases after enoxaparin use: case report and review of unfractionated and low-molecular-weight heparin-induced hepatotoxicity. Pharmacotherapy. 2001; 21:108–13.
11. Haney JS, Carter CT, Downey J, Ilic R. A severe case of idiosyncratic hepatotoxicity with unfractionated heparin. Hosp Pharm. 2021; 56:777–85.
12. AL-Mekhaizeem KA, Sherker AH. Heparin-induced hepatotoxicity. Can J Gastroenterol. 2001; 15:527–30.
13. Zaera De La Fuente C, Arribas Anta J, López-San Román A, Cañete Ruiz Á, López Durán S. Enoxaparin-induced hepatotoxicity. Gastroenterol Hepatol. 2015; 38:438–9.
14. Nozawa H, Emoto S, Sonoda H, et al. Liver injury among Japanese patients treated using prophylactic enoxaparin after colorectal surgery. Dig Dis Sci. 2021; 66:2805–15.
15. Meneveau N. Safety evaluation of enoxaparin in currently approved indications. Expert Opin Drug Saf. 2009; 8:745–54.
16. Gouin-Thibault I, Pautas E, Siguret V. Safety profile of different low-molecular weight heparins used at therapeutic dose. Drug Saf. 2005; 28:333–49.
17. Bounameaux H. Unfractionated versus low-molecular-weight heparin in the treatment of venous thromboembolism. Vasc Med. 1998; 3:41–6.
18. Solari F, Varacallo M. Low molecular weight heparin (LMWH) [updated 2022 Jul 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing;2020. [cited 2022 Jun 24]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK525957/.
19. Young E, Wells P, Holloway S, Weitz J, Hirsh J. Ex-vivo and in-vitro evidence that low molecular weight heparins exhibit less binding to plasma proteins than unfractionated heparin. Thromb Haemost. 1994; 71:300–4.
20. Hirsh J. Low-molecular-weight heparin : a review of the results of recent studies of the treatment of venous thromboembolism and unstable angina. Circulation. 1998; 98:1575–82.
21. Hahn KJ, Morales SJ, Lewis JH. Enoxaparin-induced liver injury: case report and review of the literature and FDA Adverse Event Reporting System (FAERS). Drug Saf Case Rep. 2015; 2:17.
Fig. 1.
A 41-year-old man presented with his right index finger completely amputated at the proximal phalanx level by an electrical saw (A). Replantation was performed immediately, and unfractionated heparin was administered intravenously after the operation. The patient showed elevated aspartate aminotransferase and alanine transaminase levels (220 U/L and 258 U/L, respectively), without any marked symptoms of hepatotoxicity. (B) The operation was successful with minimal sequelae.
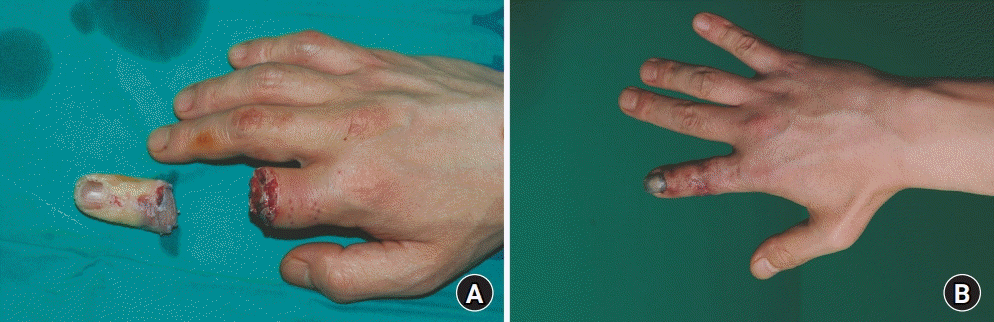
Fig. 2.
A 21-year-old man presented with complete amputation of his left thumb at the proximal phalanx and index at the mid-phalanx by an industrial cutter (A). Immediate replantation was performed, and the patient was administered low-molecular-weight heparin for perioperative anticoagulant therapy. The aspartate aminotransferase and alanine transaminase levels of this patient were normal during admission, without any evidence of hepatotoxicity. (B) Clinical photograph a year after surgery.
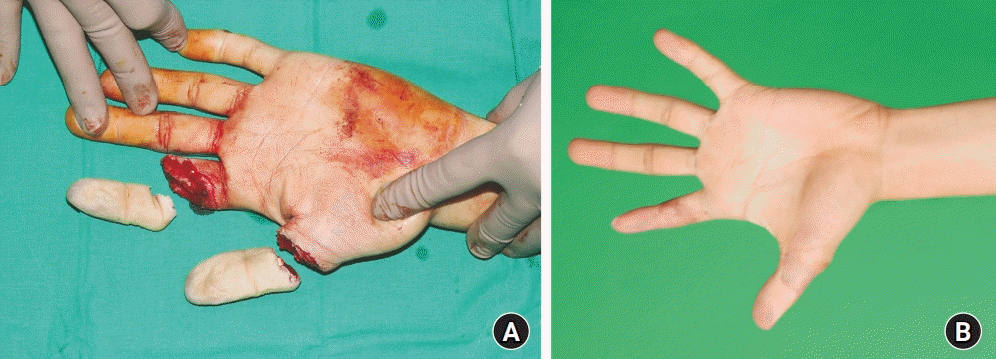
Fig. 3.
The success rates showed no difference between the two groups. LMWH, low-molecular-weight heparin; UFH, unfractionated heparin.
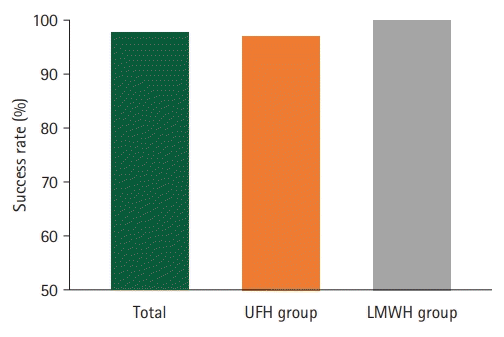
Fig. 4.
The complication rates showed significant differences between the two groups in both aspartate aminotransferase (AST) and alanine transaminase (ALT) levels. LMWH, low-molecular-weight heparin; UFH, unfractionated heparin. *p<0.05, statistically significant.
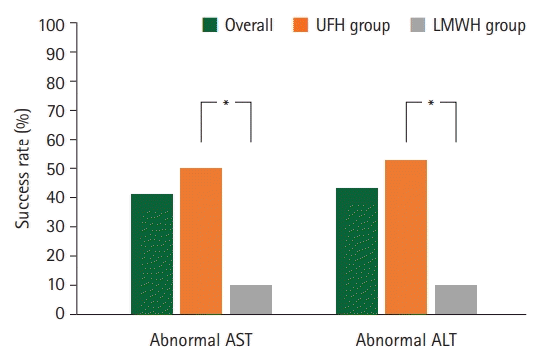
Table 1.
Demographic characteristics of the patients
Table 2.
Comparison of the success rate between groups
| Variable | Total (n=44) | LMWH group (n=10) | UFH group (n=34) | p-value |
|---|---|---|---|---|
| Success or failure | >0.999 | |||
| Success | 43 (97.7) | 10 (100) | 33 (97.1) | |
| Failure | 1 (2.3) | 0 (0) | 1 (2.9)a) |
Table 3.
Differences in liver function test results between groups
| Variable | Total (n=44) | LMWH group (n=10) | UFH group (n=34) | p-value |
|---|---|---|---|---|
| AST | 0.031* | |||
| Normal | 26 (59.1) | 9 (90.0) | 17 (50.0) | |
| Abnormala) | 18 (40.9) | 1 (10.0) | 17 (50.0) | |
| ALT | 0.027* | |||
| Normal | 25 (56.8) | 9 (90.0) | 16 (47.1) | |
| Abnormala) | 19 (43.2) | 1 (10.0) | 18 (52.9) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download