Abstract
Purpose
Wide local excision (WLE) has been used as an alternative to amputation for preserving the length of the digit in subungual melanoma (SUM). For reconstruction, a free flap could be a more suitable option than a skin graft or a local flap. We investigated the clinical value of WLE with concurrent venous free flap reconstruction in SUM located on the finger and toe.
Methods
Seventeen patients underwent WLE with a concurrent arterialized venous free flap between January 2011 and December 2015. Venous flaps were harvested from the forearm or foot dorsum and inset using a through- or against-valve type.
Results
The mean tumor area was 1.3±0.9 cm2, and the mean resection margin was 5.6±2.3 mm. In histologic analyses, the mean tumor thickness was 1.2±1.1 mm. The mean duration of the follow-up period was 75.5 months. Three patients had local recurrence and one patient had distant metastasis. Reconstruction of the fingers and toes using a venous flap was effective and could be performed without major complications. Patients were satisfied with the functional and aesthetic results.
Subungual melanoma (SUM) is a distinct form of cutaneous melanoma that accounts for 0.7% to 3.5% of all melanomas in Caucasians [1,2]. The most common histologic subtype of SUM is acral lentiginous melanoma. Notably, a higher incidence of acral lentiginous melanoma in the East Asian population results in an approximate SUM incidence of 15% to 30% among all types of melanoma [3-5]. Due to the Tyndall effect, black or brown pigmentation under the nail plate appears less dark and suspicious, causing a delay in diagnosis and treatment [2,6]. Moreover, the unique anatomy of the nail complex makes it challenging to perform an excisional biopsy and determine the tumor thickness.
Surgical treatment of SUM is typically amputation at the level proximal to the tumor. However, permanent deformity of the finger or toe due to amputation can have a substantial psychological impact on the patient [7]. Despite aggressive treatment, SUM has a poor prognosis in terms of the 5-year survival rate, which ranges from 16% to 80% [2]. To date, the treatment guidelines for SUM based on large-scale studies have not yet been defined. As an alternative, wide local excision (WLE) of the tumor without amputation has been proposed, with assurance of the tumor-free resection margin [1,8,9]. In the case of early-stage SUM, WLE with subsequent reconstruction could be considered reasonable for functional and aesthetic purposes.
Several studies have reported reconstruction of the finger or toe tissue after WLE of SUM using techniques including skin graft, local flap, distant flap, and free flap [10-16]. Although the skin graft procedure was chosen most often due to its simplicity, unfavorable events such as exposure of the phalangeal bone could occur during the surgery. When skin graft or local flap is not applicable for tissue reconstruction, free flap may be a suitable alternative. Previously, we reported the usefulness of arterialized venous free flap as a surgical option to treat skin cancers of the digits [17,18]. Venous flaps, which are readily harvested from the forearm or foot dorsal skin, can provide a three-dimensional structure to repair the defect and restore the natural contour of the fingers and toes. However, to date, there have been few studies to introduce the combination of WLE and free flap for the treatment of SUM. The aim of the current study was to investigate the clinical value of WLE concurrent with venous free flap reconstruction in SUM located on the finger and toe.
Ethics statement: The review of the medical records of all patients was approved by the Institutional Review Board (No. H-1506-109-682). Informed consent was waived due to the retrospective nature of this study.
We retrospectively reviewed patients diagnosed with a SUM located on a finger or toe between January 2011 and December 2015. Seventeen patients who received WLE concurrent with reconstruction using arterialized venous free flap were included. Clinicopathologic characteristics including sex, age at diagnosis, tumor location, area, and thickness, flap size, resection margin, histology, growth phase, and recurrence information were collected. WLE was indicated by the following: (1) absence of metastasis of regional lymph nodes and distant organs in preoperative assessment; (2) no severe secondary change of the nail complex; (3) no bone involvement of SUM identified intraoperatively; and (4) no recurrence had been noted.
Postoperative satisfaction scores regarding both functional and aesthetic aspects were obtained from reachable and surviving patients. For functional assessment, an excellent result was defined as a finger (or toe) with no pain and full range of motion (ROM) in the interphalangeal joint. A fair result was defined as a finger (or toe) with minimal pain, mild discomfort in the ROM. A poor result was defined as a finger (or toe) with constant pain, ROM impairment causing problems in daily activity. For aesthetic assessment, an excellent result was defined as a finger (or toe) with cosmetically acceptable appearance. A fair result was defined as a finger (or toe) with residual deformity. A poor result was defined as a finger (or toe) with an unacceptable appearance.
Under general anesthesia, a pneumatic tourniquet was applied to the upper arm or thigh to facilitate the venous engorgement of the forearm or foot dorsum as a donor site of the venous free flap. After outlining the tumor, a surgical margin with an average size of 5 mm was drawn. The tumor was surgically resected along the surgical margin, and the depth of the resection was at the periosteal level. During resection, efforts were made to spare the extensor terminal tendon bundle as much as possible. However, in cases where the proximal margin was further extended due to the Hutchinson sign, efforts were made to preserve the tendon as much as possible by resection with beveling [19]. For complete removal of the tumor, burring of the underlying cortical bone was conducted. If bone involvement of the tumor was suspected during the surgery, amputation proximal to the tumor was performed instead of WLE.
In the case of WLE, a design of venous flap similar to that of the defect was marked on the forearm or foot dorsum where at least three veins could be included. The flap was elevated at the suprafascial level, and several veins were harvested to provide efferent and afferent vessels for the reconstructed tissue. For the through-valve type of arterialized venous flap, the afferent vein at the distal side of the flap was anastomosed with the digital artery, and the efferent vein at the proximal side was anastomosed with the digital vein [20]. For the against-valve type, the afferent vein at the proximal side of the flap was anastomosed with the digital artery in the reverse flow direction. Subsequently, the efferent vein on the same side of the afferent vein was anastomosed with the digital vein. To reduce postoperative flap edema and congestion, additional efferent veins were anastomosed with the digital dorsal vein in some cases. After flap transfer, the donor site was repaired with direct closure or split-thickness skin graft.
Postoperatively, 20 µg of alprostadil (Eglandin; Mitsubishi Tanabe Pharma Co., Ltd., Seoul, Korea) was administered daily for 6 days. The flap was monitored by Doppler probe analysis of the pulsating flow through the anastomoses. In the event of flap congestion, heparin-soaked gauzes were applied to an intentionally de-epithelialized part of the surface of flaps until resolution.
Clinical data and satisfaction scores of the patients are listed in Table 1. All patients were free of metastasis of regional lymph nodes and distant organs on preoperative computed tomography, bone scan, and/or positron emission tomography scans. The mean tumor area was 1.3±0.9 cm2 and the mean resection margin was 5.6±2.3 mm. In histologic analyses, the mean tumor thickness was 1.2±1.1 mm and 76.5% of patients showed radial growth phase. The mean flap size was 8.1±3.2 cm2. Among various subtypes of melanoma, acral lentiginous melanoma was predominant (94.1%). Three patients had local recurrence and one had distant metastasis during the follow-up period. The mean disease-free interval and the follow-up period was 70.9 months and 75.5 months, respectively.
Reconstruction of the finger and toe using venous free flaps after WLE provided overall satisfactory results (Figs. 1, 2). Complications including flap loss or donor problems did not occur in any patient during the hospitalization period. Flap debulking was performed to resolve a size mismatch in one case. Three cases of skin defects resulted from trauma or partial necrosis after venous congestion, in which all cases were completely healed using debridement and skin grafting.
Despite a high rate of local recurrence and distant metastasis of SUM, the absence of published guidelines for surgical treatment has resulted in various surgical trials being conducted. Several reports have accumulated evidence in favor of WLE for preserving the function and aesthetics of the digits [10-16,21-25]. Tumor thickness has been widely accepted as an important factor for melanoma staging and prognosis [26]. Nakamura et al. [16] reported an acceptable local recurrence rate of WLE in patients with >0.5 mm-thick invasive SUM in situ. Recent study with 21 cadavers suggested that patients with hyponychial invasion showed a significantly greater Breslow depth, a higher rate of lymph node metastasis, distant metastasis, and shorter disease-free survival [27]. In our cases, the mean tumor thickness was 1.2 mm, ranging from in situ to 4.0 mm-thick melanoma. There was one case of local recurrence, in which the patient had a 0.4 mm-thick melanoma with hyponychial invasion at the radial growth phase and of the acral lentiginous subtype on the first toe. Cochran et al. [2] compared amputation and WLE as treatments for SUM based on previous literature. The authors determined a local recurrence rate of 2.2% for amputation and 12.2% for WLE, raising concerns over the use of WLE. Although WLE showed low rate of local recurrence in this study, it is still recommended to perform WLE only in early-stage patients to ensure a safe surgical outcome.
To complete the purpose of WLE as a digit-sparing approach, the reconstructive option should be reasonably selected. From the aesthetic perspective, free flap can provide excellent outcomes among various surgical options. In this regard, we examined the use of venous free flap reconstruction of defects occurring as a result of SUM. This technique was first introduced by Nakayama et al. [28], followed by basic research and clinical studies [29,30]. This flap has been used for resurfacing even the large defect when local flaps are not available or insufficient for coverage [31]. Given the small and thin defect after SUM tumor excision, venous flaps are preferential to conventional flaps, which tend to be bulky. Moreover, this method provides several advantages including minimal donor site morbidity, design of free-style flap, similar skin texture, and a soft tissue padding effect that is improved compared to that of a skin graft [18]. For a natural appearance, an artificial nail can be attached after flap stabilization.
Venous flaps are classified as pedicled venous flap, free venous flap, and arterialized venous flap [32,33]. Changes in blood flow cause complications that include edema, congestion, or necrosis of the flap. Yan et al. [34] summarized the algorithm of the types of venous flap and the adjunctive surgical options to improve flap survival. In arterialized venous flap, depending on the flap design, arterial blood passes rapidly through the flap, resulting in the steal phenomenon. To reduce the potential complications, the other option for venous flap, which is inset to allow reversed blood flow with against-valve type, was introduced [20]. Nevertheless, partial necrosis can develop, requiring debridement and secondary healing or additional skin graft surgery. In our study, three cases of skin grafts were performed at the defect site due to skin defects.
Limitations include the small number of patients, subjective evaluation of postoperative result, and the retrospective nature of the study. A well-designed prospective study with objective assessment such as two-point discrimination would provide further evidence for the clinical value of and indications for WLE as a treatment for SUM. However, this study includes self-reported satisfactory results with long-term consequence of WLE with a follow-up period of more than 5 years.
We investigated the clinical value of WLE concurrent with arterialized venous free flap reconstruction as a treatment for SUM affecting the fingers and toes. As a digit-sparing approach, WLE for early-stage SUM of the fingers and toes provided an acceptable local control rate and offered a safe and conservative alternative to amputation. Moreover, concurrent venous free flaps could provide excellent and satisfactory outcomes for aesthetic reconstruction.
References
1. Brodland DG. The treatment of nail apparatus melanoma with Mohs micrographic surgery. Dermatol Surg. 2001; 27:269–73.
2. Cochran AM, Buchanan PJ, Bueno RA Jr, Neumeister MW. Subungual melanoma: a review of current treatment. Plast Reconstr Surg. 2014; 134:259–73.
3. Takematsu H, Obata M, Tomita Y, Kato T, Takahashi M, Abe R. Subungual melanoma: a clinicopathologic study of 16 Japanese cases. Cancer. 1985; 55:2725–31.
4. Roh MR, Kim J, Chung KY. Treatment and outcomes of melanoma in acral location in Korean patients. Yonsei Med J. 2010; 51:562–8.
5. Nam KW, Bae YC, Nam SB, Kim JH, Kim HS, Choi YJ. Characteristics and treatment of cutaneous melanoma of the foot. Arch Plast Surg. 2016; 43:59–65.
6. Weismann K, Lorentzen HF. Dermoscopic color perspective. Arch Dermatol. 2006; 142:1250.
7. Østlie K, Magnus P, Skjeldal OH, Garfelt B, Tambs K. Mental health and satisfaction with life among upper limb amputees: a Norwegian population-based survey comparing adult acquired major upper limb amputees with a control group. Disabil Rehabil. 2011; 33:1594–607.
8. Moehrle M, Metzger S, Schippert W, Garbe C, Rassner G, Breuninger H. “Functional” surgery in subungual melanoma. Dermatol Surg. 2003; 29:366–74.
9. Anda-Juárez MC, Martínez-Velasco MA, Fonte-Ávalos V, Toussaint-Caire S, Domínguez-Cherit J. Conservative surgical management of in situ subungual melanoma: long-term follow-up. An Bras Dermatol. 2016; 91:846–8.
10. Clarkson JH, McAllister RM, Cliff SH, Powell B. Subungual melanoma in situ: two independent streaks in one nail bed. Br J Plast Surg. 2002; 55:165–7.
11. High WA, Quirey RA, Guillén DR, Munõz G, Taylor RS. Presentation, histopathologic findings, and clinical outcomes in 7 cases of melanoma in situ of the nail unit. Arch Dermatol. 2004; 140:1102–6.
12. Lazar A, Abimelec P, Dumontier C. Full thickness skin graft for nail unit reconstruction. J Hand Surg Br. 2005; 30:194–8.
13. Imakado S, Sato H, Hamada K. Two cases of subungual melanoma in situ. J Dermatol. 2008; 35:754–8.
14. Duarte AF, Correia O, Barros AM, Azevedo R, Haneke E. Nail matrix melanoma in situ: conservative surgical management. Dermatology. 2010; 220:173–5.
15. Chow WT, Bhat W, Magdub S, Orlando A. In situ subungual melanoma: digit salvaging clearance. J Plast Reconstr Aesthet Surg. 2013; 66:274–6.
16. Nakamura Y, Ohara K, Kishi A, et al. Effects of non-amputative wide local excision on the local control and prognosis of in situ and invasive subungual melanoma. J Dermatol. 2015; 42:861–6.
17. Yun MJ, Park JU, Kwon ST. Surgical options for malignant skin tumors of the hand. Arch Plast Surg. 2013; 40:238–43.
18. Park JU, Kim K, Kwon ST. Venous free flaps for the treatment of skin cancers of the digits. Ann Plast Surg. 2015; 74:536–42.
19. Kim BJ, Kim J, Hu J, Kwak Y, Kwon ST. Functional surgery for subungual melanoma: surgical tips based on histological analysis of 21 cadavers. Dermatol Surg. 2022; 48:7–11.
20. Woo SH, Kim KC, Lee GJ, et al. A retrospective analysis of 154 arterialized venous flaps for hand reconstruction: an 11-year experience. Plast Reconstr Surg. 2007; 119:1823–38.
21. Sureda N, Phan A, Poulalhon N, Balme B, Dalle S, Thomas L. Conservative surgical management of subungual (matrix derived) melanoma: report of seven cases and literature review. Br J Dermatol. 2011; 165:852–8.
22. Hayashi K, Uhara H, Koga H, Okuyama R, Saida T. Surgical treatment of nail apparatus melanoma in situ: the use of artificial dermis in reconstruction. Dermatol Surg. 2012; 38:692–4.
23. Debarbieux S, Hospod V, Depaepe L, Balme B, Poulalhon N, Thomas L. Perioperative confocal microscopy of the nail matrix in the management of in situ or minimally invasive subungual melanomas. Br J Dermatol. 2012; 167:828–36.
24. Haddock NT, Wilson SC, Shapiro RL, Choi M. Wide local en bloc excision of subungual melanoma in situ. Ann Plast Surg. 2014; 73:640–4.
25. Jo G, Cho SI, Choi S, Mun JH. Functional surgery versus amputation for in situ or minimally invasive nail melanoma: a meta-analysis. J Am Acad Dermatol. 2019; 81:917–22.
26. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009; 27:6199–206.
27. Yoo H, Kim H, Kwon ST, et al. Tumor invasion in the hyponychium is associated with distant metastasis and poor prognosis in subungual melanoma: a histologic landscape of 44 cases. J Am Acad Dermatol. 2022; 86:1027–34.
28. Nakayama Y, Soeda S, Kasai Y. Flaps nourished by arterial inflow through the venous system: an experimental investigation. Plast Reconstr Surg. 1981; 67:328–34.
29. Baek SM, Weinberg H, Song Y, Park CG, Biller HF. Experimental studies in the survival of venous island flaps without arterial inflow. Plast Reconstr Surg. 1985; 75:88–95.
30. Tsai TM, Matiko JD, Breidenbach W, Kutz JE. Venous flaps in digital revascularization and replantation. J Reconstr Microsurg. 1987; 3:113–9.
31. Yan H, Fan C, Zhang F, Gao W, Li Z, Zhang X. Reconstruction of large dorsal digital defects with arterialized venous flaps: our experience and comprehensive review of literature. Ann Plast Surg. 2013; 70:666–71.
32. Thatte MR, Thatte RL. Venous flaps. Plast Reconstr Surg. 1993; 91:747–51.
33. Yan H, Zhang F, Akdemir O, et al. Clinical applications of venous flaps in the reconstruction of hands and fingers. Arch Orthop Trauma Surg. 2011; 131:65–74.
34. Yan H, Brooks D, Ladner R, Jackson WD, Gao W, Angel MF. Arterialized venous flaps: a review of the literature. Microsurgery. 2010; 30:472–8.
Fig. 1.
Patient (case 7) with subungual melanoma on the left third finger. (A) Preoperative clinical photograph. (B) En-bloc excision of the tumor and nail complex with a 5-mm margin. (C) A venous flap harvested from the volar side of the left forearm was anastomosed with one digital artery and two digital veins. (D) Natural contour of the reconstructed finger without any complications at the 8-month follow-up. Written informed consent was obtained for publication of accompanying images.
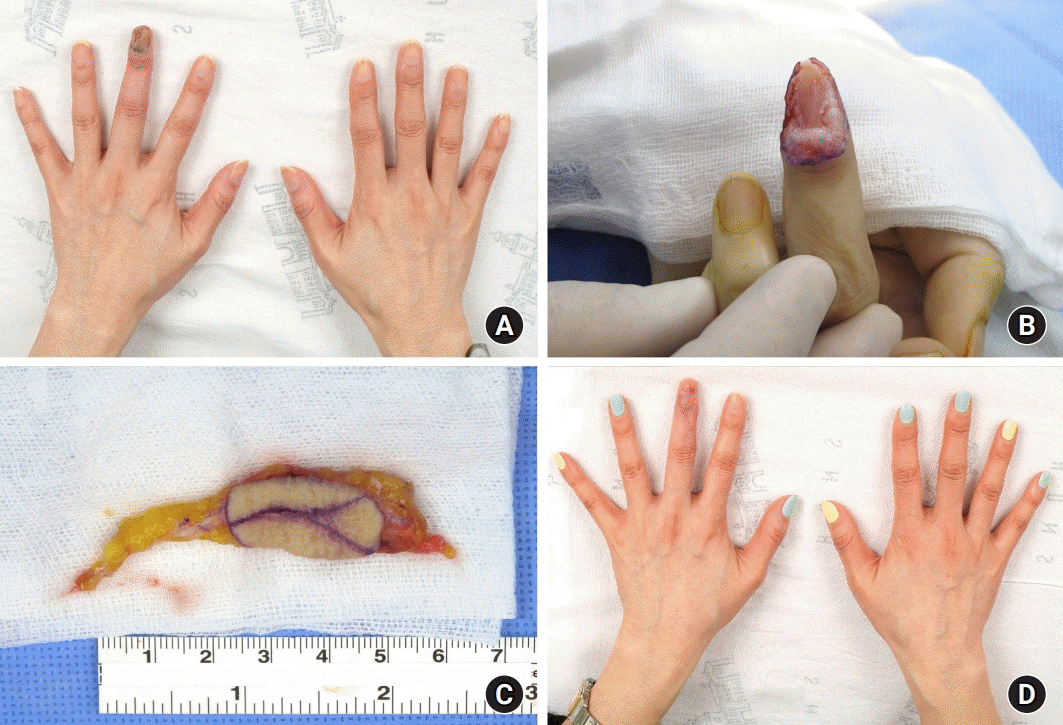
Fig. 2.
Patient (case 8) with subungal melanoma on the right first toe. (A) Preoperative clinical photograph. (B) After inset of a venous flap harvested from the dorsal side of the right foot to allow reversed blood flow with the against-valve type. (C) Clinical photograph of a skin defect that occurred at the center of the flap due to trauma 3 months after surgery. (D) Completely healed flap without further treatment at a 5-year follow-up after debridement and skin grafting for the skin defect. Written informed consent was obtained for publication of accompanying images.
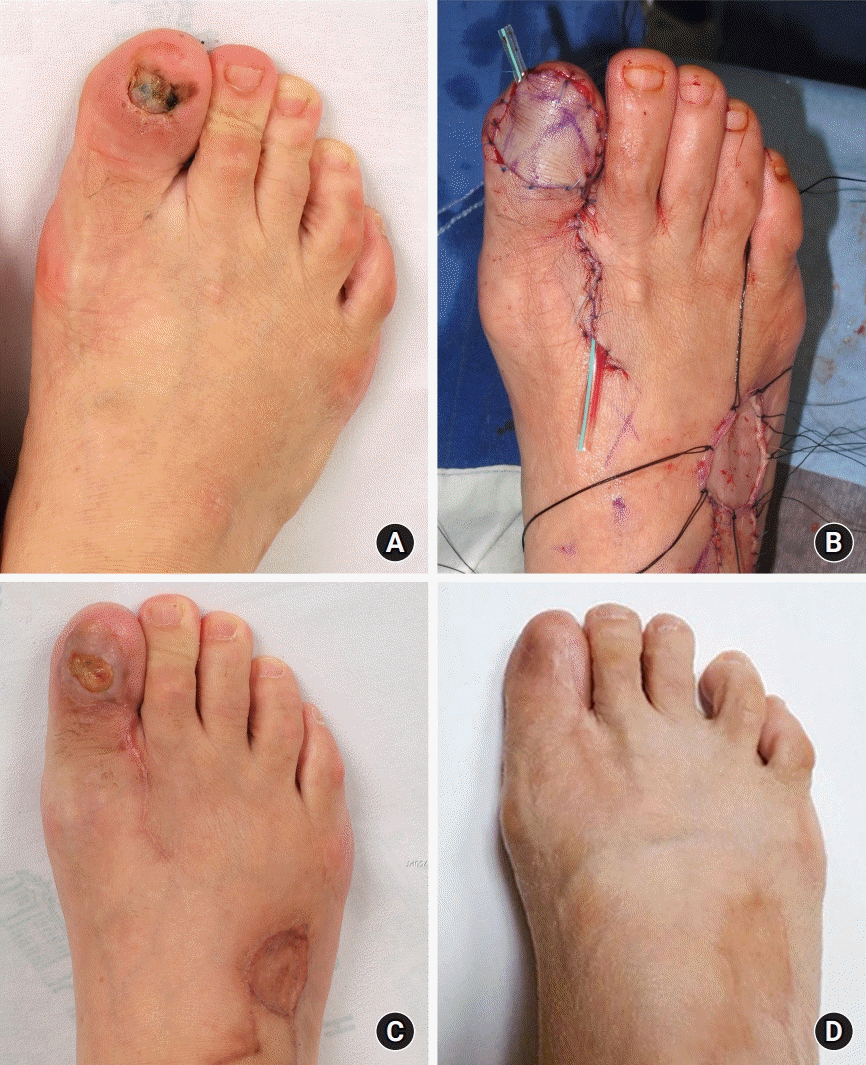
Table 1.
Summary of patients’ characteristics and satisfaction score




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download