This article has been
cited by other articles in ScienceCentral.
ABSTRACT
Variant angina, which is associated with coronary artery spam, is difficult to
recognize on routine preoperative evaluation. Coronary spasm results in
myocardial ischemia and even lethal arrhythmia in severe cases. Since patients
are unconscious and cannot complain of symptoms during general anesthesia, early
detection of such an event is difficult, and it could lead to severe bradycardia
or cardiac arrest. We report a case of a patient with previously undiagnosed
variant angina who experienced severe hypotension and ventricular fibrillation
during general anesthesia.
Keywords: Coronary vasospasm, Anesthesia, general, Hypotension, Angina pectoris, variant, Ventricular fibrillation
Introduction
Coronary artery spasm or variant angina is rare and difficult to detect on routine
preoperative evaluation. The features of ischemic episodes due to variant angina
differ from those of typical angina. Such an episode occurs when a patient is
resting or in the early morning and is not associated with exercise [
1]. Moreover, silent ischemic episodes are
frequent, and syncope is occasionally present [
2]. In severe cases, transient ST-segment elevation and arrhythmias such
as ventricular tachycardia and ventricular fibrillation can follow. Coronary artery
spasms can occur during surgery under general or regional anesthesia. Since patients
are unconscious and cannot complain of symptoms during general anesthesia, early
detection of coronary artery spasms is difficult, which could lead to severe
bradycardia or cardiac arrest. We report a case of a patient having variant angina
with severe hypotension and ventricular fibrillation during general anesthesia.
Case
A 70-year-old female was scheduled for laparoscopic cholecystectomy for chronic
cholecystitis. Her height and weight were 153.0 cm and 82.1 kg, respectively. Her
body mass index was 35.1 kg/m2. The patient had hypertension and type 2
diabetes mellitus and was a hepatitis B carrier. The patient also had prurigo
nodularis and was taking 5 mg prednisolone per day. The patient had experienced
chest pain 15 years previously and has been taking 100 mg aspirin daily since then.
The patient reported that she had not undergone any diagnostic tests, such as
angiography for coronary artery disease, following the episode of chest pain. There
were no specific findings on preoperative EKG or transthoracic echocardiogram.
After the patient was transferred to the operating room (OR), standard monitors,
including noninvasive blood pressure, EKG, pulse oxygen saturation
(SpO2), and bispectral index, were applied. The initial noninvasive blood
pressure and heart rate (HR) were 137/62 mmHg and 71 beats/min, respectively. EKG
showed a normal sinus rhythm, and SpO2 was 100%. Anesthesia was induced
uneventfully after sequential administration of glycopyrrolate (0.2 mg), midazolam
(3 mg), 1% propofol (80 mg), fentanyl (50 μg), and rocuronium (32 mg).
Following intubation, arterial cannulation was performed, and continuous arterial
blood pressure (ABP) monitoring was initiated. Results of arterial blood gas
analysis were within the normal range: potential of hydrogen (pH), 7.401; partial
pressure of carbon dioxide (pCO2), 33.6 mmHg; partial pressure of oxygen
(pO2), 260.9 mmHg; base excess (BE), −4.4 mEq/L; and arterial oxygen
saturation (SaO2): 97.7%. Anesthesia was maintained with 1%–2% sevoflurane
and intermittent fentanyl injection.
The laparoscopic procedure was conducted uneventfully for 15 min, and carbon dioxide
(CO2) insufflation was discontinued. During the surgery, EKG showed a
normal sinus rhythm, ABP ranged from 96/54 to 183/91 mmHg, and HR was 80–95
beats/min. SpO2 was maintained at > 99%, and end-tidal
CO2 tension was between 31 and 35 mmHg. Five minutes after the
beginning of wound suturing, her ABP and HR were 75/49 mmHg and 91 beats/min,
respectively, and 5 mg ephedrine was injected intravenously. However, systolic ABP
decreased to approximately 50 mmHg. After an additional dose of 5 mg ephedrine,
tachycardia with an HR of 130 beats/min was noted. Despite starting norepinephrine
infusion, ABP did not increase, and ST-segment elevation (2.5 mm) followed by
ventricular fibrillation was noted in the EKG. Chest compression was initiated
immediately, and spontaneous circulation (ROSC) was returned after one cycle (2
minutes) of cardiopulmonary resuscitation without defibrillation. Blood samples were
collected for cardiac enzyme tests, and 12-lead EKG monitoring was taken.
Despite ROSC, systolic ABP was still low (approximately 60 mmHg). Therefore, an
epinephrine infusion was started. ST-segment elevation in the territory of the left
anterior descending coronary artery was observed on 12-lead EKG (
Fig. 1). Results of post-ROSC arterial blood gas
analysis were as follows: pH, 7.283; pCO₂, 41.4 mmHg; pO₂, 342.5 mmHg;
base excess, −7.5 mEq/L; and SaO₂, 97.9%. Cardiac enzyme levels at the
time of the event were within the normal ranges (high-sensitivity cardiac troponin
T, 0.004 ng/mL and a myocardial fraction of creatine kinase, 0.7 ng/mL). At 10
minutes after the event, vital signs began to stabilize, and ST-segment elevation
was normalized. The patient was transferred to the intensive care unit under
intubation with continuous infusion of epinephrine and norepinephrine (ABP, 93/51
mmHg).
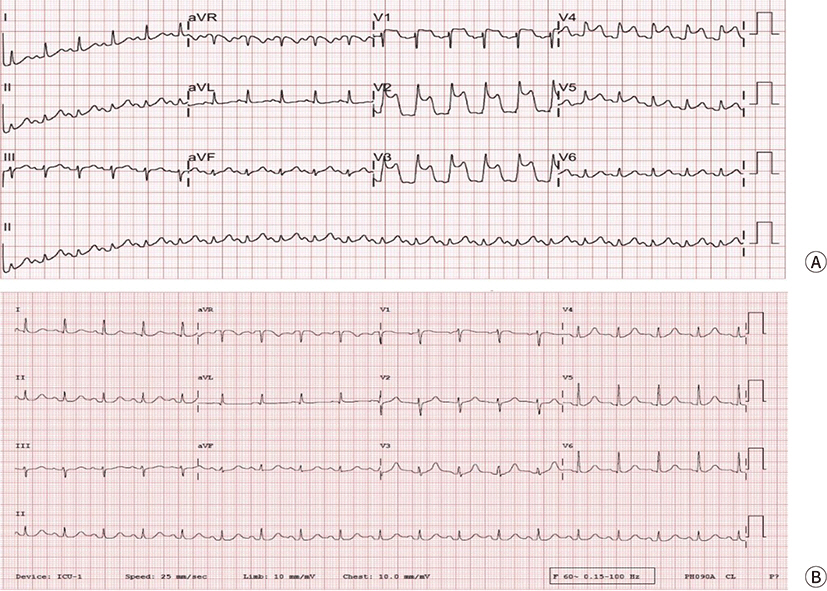
Fig. 1.
Electrocardiography following coronary vasospasm. (A) ST-segment
elevation at the leads V2–V4 was noted when the patient’s
blood pressure decreased. Immediately following this observation,
ventricular fibrillation was noted. After one cycle of cardiopulmonary
resuscitation, a return of spontaneous circulation (ROSC) was achieved. (B)
ST-segment elevation normalized shortly after ROSC.
The operation time was 45 minutes, and the anesthesia time (from arrival in the OR to
transfer to the intensive care unit) was 100 minutes. Total intake was 1000 mL of
crystalloid fluid, and total output was 100 mL (20 mL of blood loss and 80 mL of
urine output) in the OR.
Emergency coronary angiography (CAG) was performed 1 hour later, which revealed no
stenotic lesions (
Fig. 2). To avoid another
potentially refractory coronary spasm, a vasodilation test using nitroglycerin (NTG)
was performed during CAG. The right coronary artery showed reactive vasodilation
following intracoronary administration of NTG (
Fig.
2). Therefore, the patient was diagnosed with variant angina. Vital signs
stabilized after CAG, and epinephrine and norepinephrine were tapered and stopped
the next morning. The patient was extubated and transferred to the general ward the
next day and discharged 6 days later with a prescription for a calcium channel
blocker and NTG to be used when necessary.
Fig. 2.
Emergency coronary angiography. (A) and (B) No stenotic lesions were
detected in the left and right coronary arteries. (C) Dilation of the right
coronary artery was observed after intravenous administration of
nitroglycerin.

Discussion
In the present case, we experienced coronary vasospasm in a patient with undiagnosed
variant angina. The first report of the variant of angina pectoris was by Prinzmetal
in 1959 [
3]. The pain of variant angina is not
provoked by exercise or excessive workload on the heart but occurs spontaneously at
rest or during ordinary activity. Coronary vasospasm is accompanied by a
characteristic change in the EKG: significant ST-segment elevation and reciprocal
ST-segment depression [
4]. Moreover,
transmural ischemia caused by coronary artery spasms may be silent but serious,
leading to lethal arrhythmias such as ventricular tachycardia and fibrillation
[
5]. It occurs in relatively young
individuals with low coronary risk and can be confirmed by provoking local vasospasm
by vasoconstrictors such as ergonovine and acetylcholine during CAG [
1,
6,
7].
During general anesthesia, patients cannot complain of the characteristic symptoms.
Therefore, ST-segment elevation on EKG is the only clue for recognizing coronary
vasospasm. However, sometimes life-threatening arrhythmia can occur without
ST-segment change or happens too quickly before anesthesiologists can recognize the
changes in the EKG [
8,
9]. Therefore, careful vigilance and early detection of events
are critical for patient management.
Among the drugs used for anesthesia, coronary artery vasospasm has been reported to
be caused by vasoconstrictors such as ephedrine, phenylephrine, norepinephrine, and
epinephrine [
10,
11]. Hence, careful observation of any changes in the EKG after
the administration of these drugs is necessary. In the present case, the initial
decrease in ABP might not have been caused by the spasm. However, we used ephedrine
to increase the ABP, which could have caused the spasm. Another potential cause of
spasms is abdominal manipulation. At the end of CO
2 insufflation, the
surgeons pressed down the abdominal wall to pull the gas out, which could have
provoked a vagal-mediated reflex [
12,
13]. Coronary artery spasms may be affected by
the autonomic nervous system, and increased sensitivity of the vascular smooth
muscles can cause an attack [
14].
Initially, we considered the possibility of coronary artery occlusive disease due to
a definite ST-segment elevation and a history of chest pain and aspirin consumption.
In the present case, the patient had not undergone any diagnostic tests for coronary
disease despite a history of chest pain. Similarly, undiagnosed variant angina can
be overlooked when coronary vasospasm occurs during surgery. Despite the presence of
many risk factors for cardiovascular disease, the preoperative transthoracic
echocardiogram was normal, and the possibility of underlying heart disease was
believed to be low. Therefore, variant angina remained undiagnosed, and ephedrine
was used without the awareness of the potential risk of coronary artery spasm. Since
the event was diagnosed as coronary vasospasm, NTG or calcium channel blockers (CCB)
would have been helpful in relieving the artery. Generally, the primary treatment
for variant angina is NTG and CCB since both of these medications have vasodilatory
effects [
1]. NTG is a vasodilator that acts
independently of vascular endothelial cells, and CCB acts on the vascular smooth
muscles. Particularly, the response to these drugs is stronger in variant angina,
which is not associated with other factors that reduce intravascular volume, such as
vascular stenosis. Therefore, we believe that the rapid administration of these
drugs is important.
In addition, sufficient post-surgical evaluation should be conducted for an exact
diagnosis so that the patient can continue treatment if necessary. Patients should
be educated about the importance of explaining their history to future
anesthesiologists. The suspicion of coronary artery spasms can affect the early
detection of an event and the choice of drug. Therefore, detailed preoperative
history and evaluation are essential.
In conclusion, coronary artery spasms caused by variant angina can result in fatal
arrhythmias and myocardial ischemia. Therefore, caution should be exercised during
anesthesia. Furthermore, variant angina cannot be detected easily during anesthesia
since the typical symptoms and signs are masked. Therefore, careful vigilance by the
anesthesiologists, prompt response, and treatment are essential.





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download