This article has been
cited by other articles in ScienceCentral.
ABSTRACT
Extramammary Paget’s Disease (EMPD) is a rare intraepithelial malignancy
of apocrine bearing glands, which occur usually in the perianal region, vulva,
scrotum, penis and ax-illa. Most of the disease are treated by surgical
resection and the prognosis is generally good. Even though recurrent disease, it
is usually slowly progressed with good prognosis. Here we describe the case of a
70-year-old male who has presented with initially just as an EMPD component of
squamous cell carcinoma in inguinal skin, but he showed recur-rence of EMPD. The
disease has progressed rapidly, finally he died of that EMPD in 2 months of
recurrence. The purpose of this study is to report the rare case of fulminant
disease course of EMPD after recurrence.
Keywords: Paget’s disease, extramammary, Aggressive extramammary Paget’s disease
Introduction
Paget’s disease is a rare intraepithelial neoplasm. It has two sub-types, one
is mammary Paget’s disease (MPD) and the other is extramammary Paget’s
disease (EMPD) which occurs in other than mammary area. Morphology and histology of
these two subtypes are the same. The origin of EMPD is not clear but suggested that
they are originated form apocrine bearing glands, such as the perianal region,
vulva, scrotum, penis and axilla. The clinical features of EMPD can be non-specific
such as chronic eczematous cutaneous disease, therefore misdiagnosis as an
inflammatory or infective skin condition is common. Its final diagnosis is often
delayed for a long time. The prognosis of this disease entity is variable according
to clinical situation and recent analysis of 2,000 EMPD patients showed that EMPD in
scrotal lesion, concurrent malignancy, metastatic lesion, old age and male are the
risk factors for mortality [
1–
4]. Chan et al. reported that 8.3% of EMPD
patients showed concurrent malignancy in colon and prostate [
5]. Sometimes, there are co-existing squamous cell carcinoma in
situ in the skin [
6].
Surgical resection with negative margin is the considered to be the standard of care
in EMPD, other options include imiquimod 5% topical cream, modified peripheral Mohs
surgery and radiation therapy [
7,
8]. Although recurrence is common, the disease
has a slow progression and paucity metastasis, so the prognosis is generally
good.
Here, we describe a case of rapid progressed EMPD patient, who showed fulminant
disease course in two months.
Case Report
A 70-year-old man presented with discomfort in the right inguinal area. He had a
history of skin malignancy in the right inguinal area three years ago and treated
with surgical resection (wide excision and split thickness skin graft from thigh).
The histopathological study at that time showed mostly squamous cell carcinoma
(3×2.5 cm sized with depth of 4 mm invasion) and focal EMPD component (
Fig. 1). During the last three years, he did not
visit the clinic. This time he visited the hospital due to right inguinal
discomfort. In physical examination, he showed palpable right inguinal lumps without
obvious skin change. CT scan showed difuse skin thickening and subcutaneous
infiltration at right inguinal and pubic area with mild lymph node enlargements.
There was no other visceral lesion in the CT scan (
Figs. 2A,
2B). Blood test showed no
specific finding except for a mild CEA elevation (10.4 ng/mL, reference range
0–5.0 ng/mL). He underwent a punch biopsy in right inguinal lymph nodes area.
The pathology showed the consistent findings of EMPD and immunohistochemical stains
showed CK7 (+), CEA (+) and HER2 SISH (–). Further groin dissection was
planned. However, he wanted to postpone surgery due to private reasons.
Fig. 1.
Histopathologic images of skin lesion. (A) Initial skin biopsy of pubic
area, the epidermis shows the Paget cells with abundant clear cytoplasm with
intracellular and extracellular mucin production (H&E, ×200).
(B) Positive staining for CK7 (IHC, ×200). (C) Positive staining for
CEA (IHC, ×200). IHC, immunohistochemical.
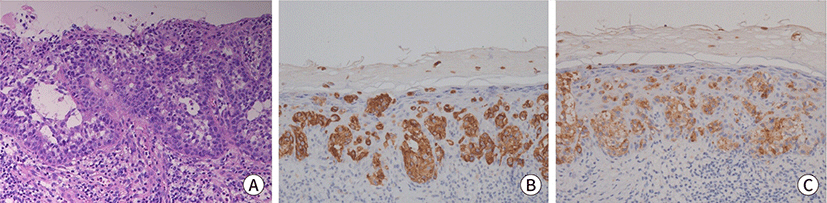
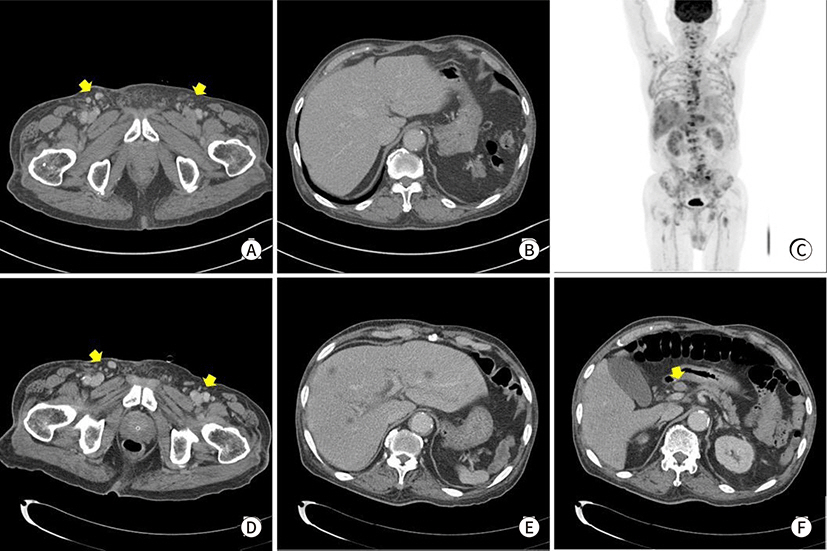
Fig. 2.
Radiologic findings. (A) Abdomen CT reveals diffuse skin thickening and
subcutaneous infiltration at right inguinal and pubic area with
lymphadenitis at recurrence (arrows), (B) no other visceral lesion at
Abdominal CT of recurrence, (C) FDG-PET CT of after 2 months. (D–F)
Abdominal CT after 2 months.
However, two months later, he visited the clinic again due to severe back pain. Spine
MRI showed several metastatic lesions in the lumbar spine, sacrum and pelvic bones.
The blood test showed anemia with hemoglobin level of 6.1 g/dL and elevated CEA
level (21.2 ng/mL) compared with results obtained two months ago. Endoscopic
findings including colonoscopy and duodenofiberscopy showed no bleeding tendency or
no specific lesion. Subsequent CT scan showed multiple hepatic metastasis, various
lymph nodes enlargements, and bony metastasis in abdomen. However no significant
change of inguinal lymphadenopathies was documented as EMPD two months ago. FDG PET
scan showed similar findings (
Figs.
2C–
2F). At the time of
admission, the level of bilirubin was in normal range, but suddenly he showed
jaundice with increasing serum bilirubin level. He underwent liver biopsy and the
pathologic results showed mmetastatic carcinoma from the previous EMPD with the same
immunohistochemical results of CK7 (+), CEA (+), GCDFP-15(+), HER2 (–) (
Fig. 3). We planned chemotherapy for the
metastatic EMPD. However just a few days, he showed rapid clinical deterioration
with hepatic failure (
Fig. 4). Eventually, on
the 12nd admission day, he died of the disease.
Fig. 3.
Histopathologic images of liver lesion. (A) Liver biopsy, the Paget cells
clusters with clear cytoplasm in hepatic sinusoid (H&E, ×100).
(B) Positive staining for CK7 (IHC, ×200). (C) Positive staining for
CEA (IHC, ×200). IHC, immunohistochemical.
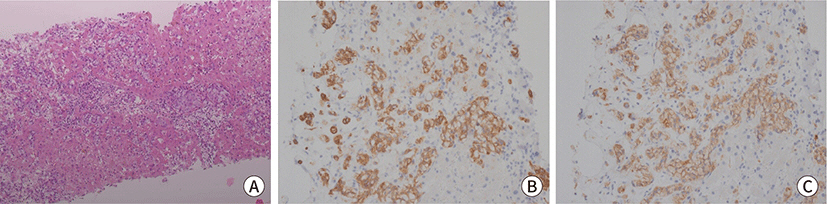
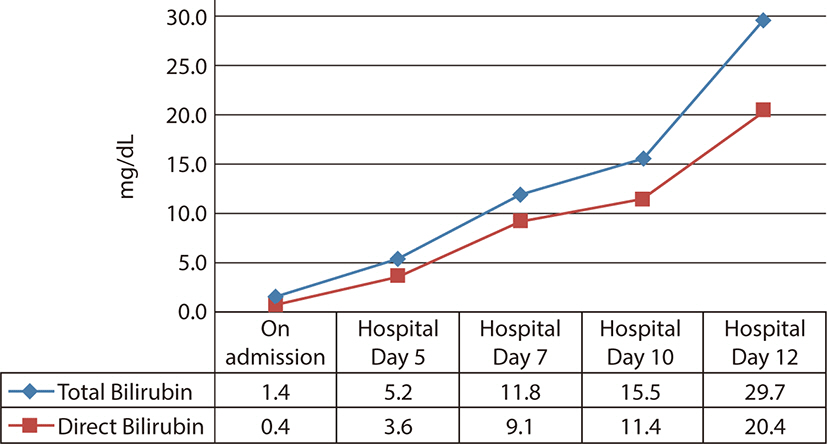
Fig. 4.
The increment of bilirubin within 2 weeks.
This is the first report about a fulminant disease course of a metastatic, recurrent
EMPD to this time in our country.
Discussion
EMPD is a rare slow-growing cutaneous adenocarcinoma that presents as an
erythematous, eczematous plaque outside the mammary gland. This neoplastic lesion is
often delayed before diagnosis, but usually resulted good prognosis. The range of
EMPD recurrence is very wide, for the example, the recurrence of EMPD in vulva
varies from 12% to 61% [
5,
9]. On multiple variate analysis, dermal
invasion, lymph node metastasis and elevated serum CEA associated with poor
prognosis have been reported [
10]. The
analysis from large scale patients showed that surgery is a protective factor and
radiation is a risk factor for EMPD survival [
4]. Although the invasion of dermis is uncommon, if once the EMPD
invades into the dermis, that could get metastatic characteristics [
11]. Interestingly, Chanda et al. reported that
46% of patients with EMPD associated with an underlying carcinoma developed
metastatic disease, compared to 18% of patients with a non-invasive disease [
12]. In a large study of 1,439 patients with
invasive EMPD, disease specific five year survival was 94.9% (95% CI
92.7%–96.5%) for localized disease, 84.9% (95% CI 77.4%–90.0%) for
regional disease and 52.5% (95% CI 29.3%–71.3%) for distant disease. For
patients diagnosed with distant spread the outlook was still favorable since more
than half of all patients were alive at 5 years of observation [
13].
In the case of our patient, he had the risk factor of concurrent skin squamous cell
carcinoma, his EMPD presented just as a minor component of that skin squamous cell
carcinoma. However, after relapse, EMPD was the only lesion except squamous cell
carcinoma. Meanwhile, the most interesting things in this case is that he rapidly
progressed to death within three months after diagnosis of relapse. Actually this
patient visited EMPD recurrence after 3 years of primary treatment, and there was an
evidence of dermal invasion such as subcutaneous infiltrative lesions in the CT scan
without visceral or distant metastasis. However, two month’s break resulted
in distant failure and finally death. Thus, the findings of regional node
involvement mimicking lymphadenitis would be a grave sign for rapid distant
progression. Recently one study has shown that systemic treatment of EMPD in the
disease is metastatic, especially some EMPD showed HER2 positivity and anti-HER2
therapy could result in a durable response and durable survival [
14].
It is still uncertain whether long standing local recurrent disease will transform to
invasive form. Chang et al. reported that chemokine receptors, CXCR4 and CXCR7 are
associated with poor outcome and that they can be used as prognostic biomarkers
[
15]. In the recent basic and
translational research, many other disease mechanisms are under elucidation
including RAS-RAF-MEK-ERK signaling, PI3K-AKT-mTOR signaling and androgen-AR
signaling. In the future, diagnostic and therapeutic application of this research
outcome would make the management of EMPD much better.
Conclusion
EMPD is an intraepithelial malignancy, which shows recurrence and occasional
metastasis, with usually good prognosis. Although several studies reported this
disease entity, the focus was on the initial skin lesions with limited experience of
disease locations and surgical outcomes. This study is the first report of a poor
clinical outcome in three months of presentation involving aggressive deterioration
of metastatic EMPD. The findings aid physicians in selectively managing EMPD
patients, especially those diagnosed with the disease concomitant with squamous cell
carcinoma of skin, for enhanced clinical out-comes.
Acknowledgments
We are grateful to Dr. Min Sun Cho in the Pathology department of Ewha Womans
University hospital for her contribution to this work.
Ethics Approval and Consent to Participate
References
1. Tanaka VDA, Sanches JA, Torezan L, Niwa AB, Festa Neto C. Mammary and extramammary Paget's disease: a study of 14
cases and the associated therapeutic difficulties. Clinics. 2009; 64(6):599–606. DOI:
10.1590/S1807-59322009000600018. PMID:
19578667. PMCID:
PMC2705154.

2. Lopes Filho LL, Lopes IMRS, Lopes LRS, Enokihara MMSS, Michalany AO, Matsunaga N. Mammary and extramammary Paget's disease. An Bras Dermatol. 2015; 90(2):225–231. DOI:
10.1590/abd1806-4841.20153189. PMID:
25830993. PMCID:
PMC4371672.

4. Yao H, Xie M, Fu S, Guo J, Peng Y, Cai Z, et al. Survival analysis of patients with invasive extramammary Paget
disease: implications of anatomic sites. BMC Cancer. 2018; 18(1):403. DOI:
10.1186/s12885-018-4257-1. PMID:
29636019. PMCID:
PMC5894213.
5. Chan JYW, Li GKH, Chung JHP, Chow VLY. Extramammary Paget’s disease: 20 years of experience in
Chinese population. Int J Surg Oncol. 2012; 2012:416418. DOI:
10.1155/2012/416418. PMID:
22500220. PMCID:
PMC3303748.
6. Baldovini C, Betts CM, Reggiani C, Reggiani M, Foschini MP. Ultrastructural examination of a case of pagetoid Bowen disease
exhibiting immunohistochemical features in common with extramammary Paget
disease. Am J Dermatopathol. 2015; 37(7):e83–e86. DOI:
10.1097/DAD.0000000000000123. PMID:
24786579.
7. Guerra R, Misra S. Management of extramammary Paget's disease: a case report
and review of the literature. Case Rep Dermatol Med. 2013; 2013:436390. DOI:
10.1155/2013/436390. PMID:
24349803. PMCID:
PMC3848195.
9. Nitecki R, Davis M, Watkins JC, Wu YE, Vitonis AF, Muto MG, et al. Extramammary Paget disease of the vulva: a case series examining
treatment, recurrence, and malignant transformation. Int J Gynecol Cancer. 2018; 28(3):632–638. DOI:
10.1097/IGC.0000000000001189. PMID:
29324542.

10. Zhang N, Gong K, Zhang X, Yang Y, Na Y. Extramammary Paget's disease of scrotum—report of
25 cases and literature review. In : Droller MJ, editor. Urologic oncology: seminars and original investigations. Amsterdam: Elsevier;2010. p. 28–33. DOI:
10.1016/j.urolonc.2008.07.002. PMID:
18805708.
12. Chanda JJ. Extramammary Paget's disease: prognosis and relationship
to internal malignancy. J Am Acad Dermatol. 1985; 13(6):1009–1014. DOI:
10.1016/S0190-9622(85)70254-X.

13. Karam A, Dorigo O. Treatment outcomes in a large cohort of patients with invasive
extramammary Paget's disease. Gynecol Oncol. 2012; 125(2):346–351. DOI:
10.1016/j.ygyno.2012.01.032. PMID:
22293043.

14. Ichiyama T, Gomi D, Fukushima T, Kobayashi T, Sekiguchi N, Sakamoto A, et al. Successful and long-term response to trastuzumab plus paclitaxel
combination therapy in human epidermal growth factor receptor 2-positive
extramammary Paget's disease: a case report and review of the
literature. Mol Clin Oncol. 2017; 7(5):763–766. DOI:
10.3892/mco.2017.1422. PMID:
29181166. PMCID:
PMC5700281.

15. Chang K, Li GX, Kong YY, Shen XX, Qu YY, Jia ZW, et al. Chemokine receptors CXCR4 and CXCR7 are associated with tumor
aggressiveness and prognosis in extramammary Paget disease. J Cancer. 2017; 8(13):2471–2477. DOI:
10.7150/jca.19127. PMID:
28900484. PMCID:
PMC5595076.








 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download