INTRODUCTION
The skin is the largest organ of the body, accounting for approximately 15% of adult body weight. Its main functions include protecting the body from external physical, chemical, and biological hazards, preventing excessive water loss, and thermoregulation. Together, skin and its associated structures constitute the integumentary system. The skin is composed of three layers: the epidermis, dermis, and subcutaneous tissue (1). Keratinocytes, which produce keratin, are the predominant cell type of epidermis. The dermis is primarily composed of collagen, a structural fibrillar protein. The depth of these layers varies depending on their location (2).
Although the first line of defense against invading microbes is innate immunity, the immune system of the skin has elements of both innate and adaptive immunity (3). This feature of innate immunity is programmed in the DNA and consists of a nonspecific response provided by pathogen recognition receptors (PRRs), including toll-like receptors (TLRs) and nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs) (4, 5). TLR engagement with exogenous pathogen-associated molecular patterns triggers the release of proinflammatory cytokines and the recruitment of immune cells (4). Endogenous damage-associated molecular patterns (DAMPs) are TLR and NLR activators (6). DAMPs act as alarm signals to the immune system when it recognizes an injury (6). NLRs which are the highly conserved cytosolic PRRs have important roles in monitoring the intracellular environment for signals of infection, toxic materials, and metabolic disturbances (5). When NLRs detect these danger signals, they oligomerize into substantial macromolecular scaffolds and quickly launch effector signaling cascades to regain homeostasis (7).
Particulate matter (PM) is a heterogeneous mixture of solid and liquid particles suspended in the air (8). PM is classified into three categories based on size: PM0.1 (0.1 μm, ultrafine particles), PM2.5 (2.5 μm, fine particles), and PM10 (10 μm, inhalable particles) (8). Air pollution is a significant environmental issue that endangers global health. PM2.5 is toxic and increases the risk of cardiovascular and respiratory illnesses (9, 10, 11). The skin protects the body against environmental pollutants. The skin is an organ that is maximally exposed to the external environment; therefore, environmental pollutants can be easily absorbed through the skin, causing local and systemic damage (12).
Recent epidemiological research has found that PM2.5 influences the development and exacerbation of inflammatory skin conditions, such as acne, atopic dermatitis (AD), and psoriasis (13, 14, 15, 16). Eczema and itching of the skin are the hallmarks of AD and are becoming more common worldwide (17, 18, 19, 20, 21, 22, 23).
The stream of work supporting the role of ambient PM on the skin immune system has grown in recent years. The growing body of evidence is based on both in vitro and in vivo analysis (24). The objective of this review is to assess the most recent research on how PM affects the skin’s immune system.
Go to : 
STRUCTURE AND FUNCTION OF THE SKIN
Human skin, which has three layers (the epidermis, dermis, and hypodermis), serves as its outermost layer of protection (25). The epidermis is the outermost layer composed of stratified squamous epithelium, including an underlying basal lamina (25). It protects the body’s surface and acts as a barrier against infection (25). The epidermis contains Langerhans cells (LCs), CD8+ resident memory T cells (TRM), γδ T cells, and keratinocytes. Starting with the outermost layer, the epidermis can be further subdivided into stratum corneum (SC), granulosum, spinosum, and basel (26). Additionally, the stratum lucidum is found only on the soles of the feet and palms of the hands. The keratinized layer of skin allows for water retention while excluding harmful substances and pathogens, creating a natural barrier to infection (26, 27). Keratinocytes are primarily responsible for maintaining the skin’s physical barrier by integrating with the SC. The SC is a lipid-rich intercellular matrix that surrounds the corneocyte layers, which are connected by corneodesmosomes (26, 28). The corneocyte envelope is comprised of structural proteins, including loricrin, involucrin, filaggrin, and tiny proline-rich proteins, and is crucial for preserving the structural stability of the skin barriers (28). The dermis is a network of fibrous, filamentous, and amorphous connective tissues that allows the recruitment of fibroblasts, mast cells, macrophages, neural networks, and vascular tissues (29). In response to various stimuli, other blood-borne cells, such as lymphocytes, plasma cells, and leukocytes, are recruited to the dermis (30). The dermis, which comprises the majority of the skin, is responsible for fluidity, elasticity, and tensile strength. It protects the body from mechanical damage, stores water, assists in thermoregulation, and possesses sensory receptors (30). The interaction between the dermis and epidermis preserves the properties of both tissues. The dermal-epidermal interface and epidermal appendages are created through the cooperation of these two regions throughout development (29, 30). As wounds heal, they work together to repair and remodel the skin. Although the dermis lacks a precise differentiation sequence that mirrors epidermal differentiation, the structure and organization of connective tissue components are discernible at different depths (31). The third layer is the subcutaneous (or hypodermis) tissue composed of fibroblasts, adipose cells, and macrophages. It supplies the body with a source of energy (29, 30, 31).
Go to : 
THE SKIN’S IMMUNE SYSTEM
The epidermis and dermis contain various types of innate immune cells, including macrophages, mast, dendritic (DCs), and innate lymphoid cells (32, 33). Keratinocytes are not only barrier progenitors, but also members of the innate immune system. When activated, they express a variety of PRRs and produce cytokines including tumor necrosis factor-α (TNF-α), thymic stromal lymphopoietin, interleukin (IL)-33, and other IL-1 family members (34). These cytokines are necessary to recruit immune cells to the skin and activate skin-resident immune cells (33, 34).
LCs are a unique subpopulation of professional antigen-presenting cells found in the epidermis. According to recent research, LCs are a subpopulation of tissue-inhabiting macrophages that gain a dendritic cell appearance and function after differentiating in the skin (33). In addition to LCs, two distinct subsets of T cells are present in the mouse epidermis: γδ T cells and TRM. γ´ T cells, a subgroup of immune cells, are constantly present in the mouse epidermis but not the human epidermis (35). The skin comprises a diverse community of T lymphocytes that contribute to conventional immune responses, such as effector T and regulatory T (Treg) cells, both of which have been detected in numerous infections and inflammatory illnesses. T helper type 17 (Th17) cells, which is a subset of CD4+ T cells, produce IL-17, IL-21, IL-22, and IL-26 (36, 37). Th17 cells, particularly those in the skin, are critical for epithelial immune responses (36, 37). In the presence of IL-1 and tumor growth factor (TGF)-β, Th17 cells differentiate in response to cytokines IL-21, IL-23, and IL-6 via activation of the signal transducer and activator of transcription 3 (38).
The dermis is under constant surveillance by macrophages, neutrophils, and T cells for pathogens. However, a large number of additional immune cells can be recruited to the dermis in response to any inflammatory stimulus (39).
Go to : 
PARTICULATE MATTER
PM is produced through the conversion of gaseous precursors such as ammonia, sulfur dioxide, volatile non-methane organic compounds, and nitrogen oxides, emitted from both natural and anthropogenic sources (40). Examples of anthropogenic sources include industrial activities, agricultural operations, pavement erosion due to road traffic, tire and brake abrasion, and fuel combustion (lignite, biomass, heavy oil, and coal). In contrast, spindrifts, wildfires, volcanoes, and dirt cyclones are examples of natural sources of PM (40, 41). Inorganic ions, including ammonium, calcium, chloride, magnesium, potassium, and sodium, are typically detected as chemical components of PM. This PM contains metals (Cu, V, Zn, Ni, and Cd), inorganic and organic carbon, crustal materials, particle-bound fluids, and polycyclic aromatic hydrocarbons (PAHs) (40. 41. 42).
Air pollution releases PM and is, thus, a global issue that has raised significant public health concerns (43). The link between PM production and public health problems has received considerable attention in recent years (43). Several toxicological and epidemiological studies suggested that PM is linked to detrimental biological effects on multiple main organs, including the cardiovascular and immunological systems (44, 45). The skin, similar to the respiratory tract, is the primary tissue exposed to ambient contaminants and serves as an interface between the body and the surrounding environment (46).
Go to : 
EPIDEMIOLOGY OF PM-RELATED DISEASES
Organic compounds in PM (i.e., PAHs) are extremely lipophilic and can easily permeate the skin (47). PAHs activate the aryl hydrocarbon receptor (AhR), a ligand-dependent transcription factor found in melanocytes and keratinocytes (47). PAH activation of AhR stimulates the production of intracellular reactive oxygen species (ROS) by upregulating cytochrome P450 (CYP1A1) expression, which is involved in xenobiotic metabolism (48). Oxidative stress appears to be a common mechanism of PM-induced damage (48, 49, 50). Both clinical dermatologists and researchers have recently focused on the influence of PM on the skin, recognizing ambient PM2.5 as a critical risk factor for skin diseases (23, 51, 52). PM has therefore been shown to increase allergic dermatitis and eczema symptoms in children and to promote inflammatory illnesses and skin malignancies (23, 51, 52).
AD is a persistent inflammatory skin condition that primarily affects young people. According to an international survey, the prevalence of AD in children between the ages of 6 and 7, and 13 and 14 years is rising worldwide (53). Both epidermal barrier abnormalities and immunological dysregulation contribute to AD pathogenesis (54). According to the “outside-in” theory, breakdown of the skin barrier followed by penetration of allergens is the leading cause of AD development (55). The “inside-out” concept states that immunologic predisposition causes epidermal barrier abnormalities, which leads to the development of AD (56). Accordingly, increased concentrations of T helper type 2 (Th2) cytokines, such as IL-13 and IL-4, suppress epidermal protein expression, resulting in skin barrier abnormalities (57, 58, 59).
Elevated concentrations of PM2.5, PM10, and PM0.1 exacerbate AD symptoms, such as itching in children who already have the disease (60). Furthermore, short-term PM0.1 nitrogen dioxide exposure mostly disrupts epidermal barrier functions, causing water loss and exacerbating AD symptoms (61). Further air pollution may worsen the condition (62, 63).
Go to : 
PM EFFECTS ON THE SKIN’S IMMUNE SYSTEM
Through both direct and indirect mechanisms, PM has been shown to compromise the skin barrier and trigger the induction of an inflammatory cascade in human skin (24). The most important findings regarding the effect of PM on the skin’s immune system are shown in Fig. 1.
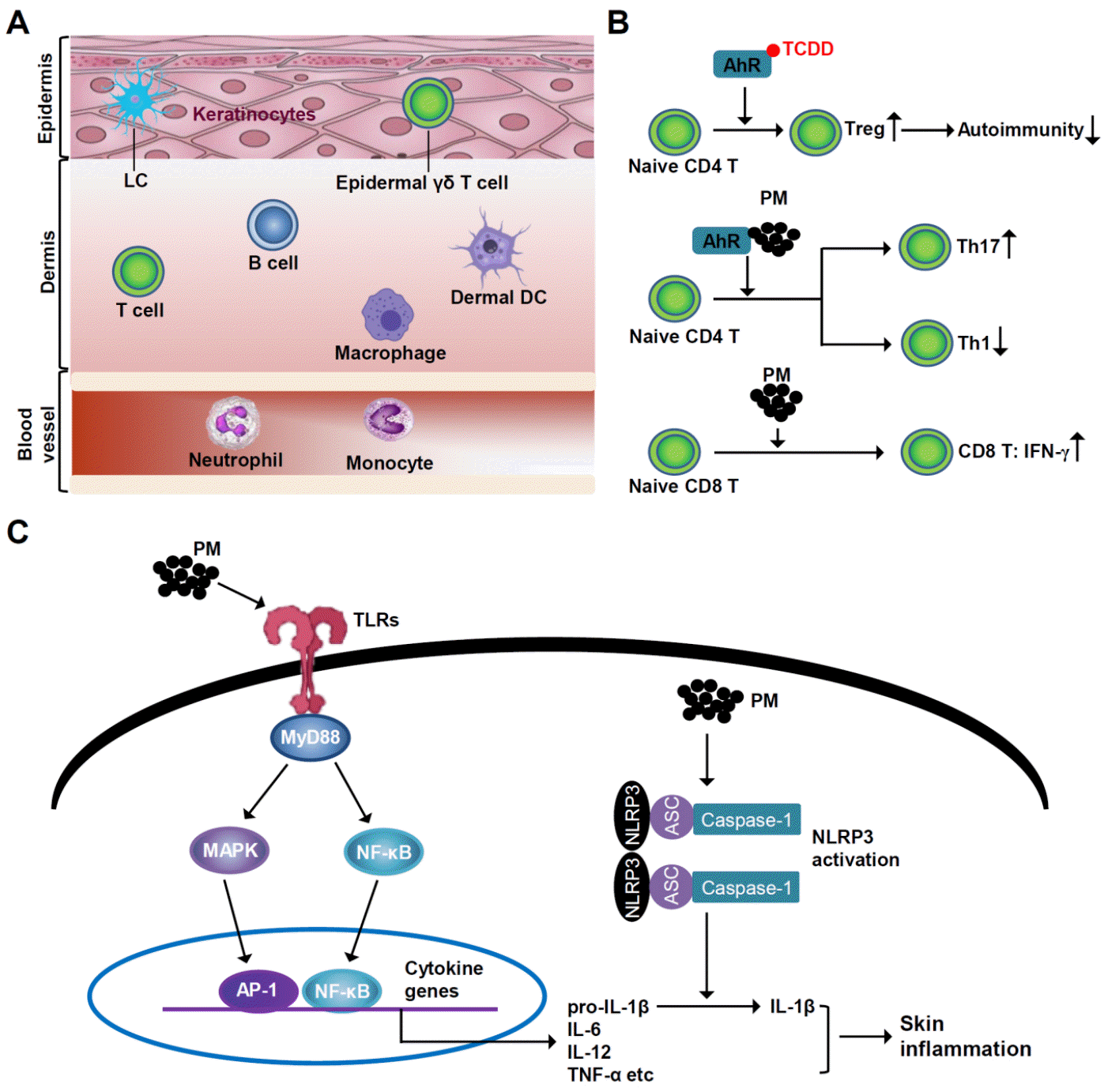 | Fig. 1Effect of PM on the skin’s immune system. (A) Normal skin immune system. Keratinocytes, γδ T cells and LCs reside in the epidermis. In the dermis, macrophages, DCs, B and T cells are present, contributing to immune responses. (B) Effect of PM on T cell differentiation. PM modulates differentiation of Th17 and Treg cells via AhR activation. AhR engagement with TCDD, an air pollutant, leads to increase in Treg differentiation and hence decreases autoimmunity. PM can also promote the polarization of Th17 via AhR. PM-exposed DCs decrease the proliferation of CD4+ Th1, and the vice versa increase the production of IFN-γ from CD8+ T cells. (C) Effect of PM on intracellular inflammatory signaling. PM binding to TLRs triggers signaling via MyD88 and downstream molecules, which leads to nuclear translocation of NF-κB, where it binds to the promoter region of interleukins, including IL-6. In addition, NLRP3 inflammasome is also activated on PM exposure. Consequently, there is increase in pro-inflammatory cytokines secretion, including IL-1β, which leads to skin inflammation. AhR, aryl hydrocarbon receptor; AP-1, activator protein 1; DC, dendritic cell; IFN, interferon; IL, interleukin; LC, Langerhans cell; MyD88, myeloid differentiation primary response 88; NF-κB, nuclear factor-κB; NLRP3, NOD-, LRR- and pyrin domain-containing protein 3; PAH, polycyclic aromatic hydrocarbon; PM, particulate matter; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; Th, T helper cell type; TLR, toll-like receptor; Treg, regulatory T cell. |
Effect of PM2.5 on differentiation and effector functions of T cells
Studies have demonstrated a link between PM2.5 and the onset and progression of skin conditions (64). Air pollution’s ability to induce pro-inflammatory immune responss remains a persistent problem. Air pollution can alter immune responses while enhancing Th2 and Th17 adaptive immunological responses, as observed in allergies (65). By altering the balance of Th1/Th2 and Th17 cells, PM2.5 harms the human immune system (65, 66, 67). AhR controls the production of Th17 and Treg cells (66, 67, 68). AhR engagement with the air pollutant 2,3,7,8-tetrachlorodibenzo-p-dioxin generates Treg cells and decreases experimental autoimmune encephalomyelitis (EAE), whereas AhR stimulation by 6-formylindolo[3,2-b]carbazole, aggravates EAE by both interfering with Treg cells differentiation and increasing Th17 cells differentiation (66, 67). Thus, AhR modulates Th17 and Treg cells differentiation in a ligand-dependent manner (67, 69). IL-17 plays a crucial role in the pathogenesis of inflammatory diseases. By promoting Th17 polarization and activation through an AhR-dependent mechanism, PM2.5 can both directly affect T cell activity through its organic structure and can worsen disease severity (70, 71, 72). An increase in IL-22, IL-23R, and IL-17A expression is associated with induction of Th17 differentiation (70). Increased expression of IL-22 and IL-23R has been recently proposed as a marker for pathogenic Th17 cells that can cause autoimmunity in animal models (71). However, PM has a more nuanced effect on DCs and lymphocytes. Urban particulate matter treatment of DCs with naive T cells increased the proliferation of CD4+ T cells in a mixed leukocyte reaction but with a lower percentage of interferon-γ (IFN-γ)-producing effector Th1 cells (73). A subsequent study using naive CD8+ T cells in mixed leukocyte responses revealed that PM-treated DCs increased CD8+ T cell production of IFN-γ, indicating that PM-exposed DCs have different immunomodulatory effects on CD8+ and CD4+ T cells (73).
PM2.5-induced NF-κB and MAPK pathways
Pollutant components activate cells via several cellular detection systems, such as TLRs and PAH-sensing pathways, including AhR (74). Consequently, pro-inflammatory intracellular signaling cascades, such as the NF-κB and MAPK pathways, are activated. ROS can induce toxicity by directly affecting cellular proteins and DNA by activating pro-inflammatory pathways (75). The intracellular danger signals produced by oxidative stress can trigger inflammasome reactions (76, 77).
PM-induced synthesis of adhesion molecules and proinflammatory cytokines controls increased inflammation (77). Transcription factors regulate the production of inflammatory signals. For example, NF-κB, a frequent target of PM, modulates pro-inflammatory cytokines (TNF-α, macrophage inflammatory protein-1α, IL-1β, -2, -6, -8, -12, GM-CSF), adhesion molecules (vascular cell adhesion molecule-1, E-selectin, and intercellular adhesion molecule-1), and immune receptors (T-cell receptor β chain, platelet-activating factor receptor, and IL-2 receptor) (77). In human epithelial cells, PM can activate NF-κB, but not AP-1, to induce cytokine transcription (77, 78, 79). NF-κB activation is controlled by numerous signaling mechanisms. Both the MAPK and phosphatidylinositol 3-kinase (PI3K)/Akt-mediated signaling pathways play a significant role (80). Since Akt is a PI3K substrate, its complete activation is achieved by Ser-473 and Thr-308 phosphorylation (80). In response to different stimuli, NF-κB activity is mediated by MAPKs, specifically ERKs and p38 MAPK (81). However, in JB6 P+ mouse epidermal cells, they play a minor role in PM-induced NF-κB activation (82). Additionally, PM2.5 binding to TLR5 triggers intracellular signaling via MyD88. MyD88 and TLR5 signaling then activate downstream signaling molecules, which leads to nuclear translocation of NF-κB, where it binds to IL-6’s promoter (77). Upon PM exposure to mouse skin, the epidermal layer thickens, indicating an inflammatory cascade and neutrophil infiltration (83). Topical PM treatment leads to dermal cell infiltration, elevated expression of the functional homolog of IL-8, and increased epidermal thickness in animal models with compromised skin barriers (83). This effect suggests that PM is correlated with the symptoms of skin conditions, such as atopic eczema (AE), which has a disrupted skin barrier (24, 83). Additionally, topical administration of the soluble PAHs from diesel fuel exacerbates lesion formation and increases immunoglobulin E levels in AE mouse models (84).
PM-induced NLRP3 inflammasome activation
Epithelial cells to respond to PM by producing NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome responses, including IL-1β (85). The NLRP3 inflammasome is the primary innate immune mechanism that generates active caspase-1 and IL-1, which are implicated in the sterile inflammatory response; thus, mitochondria are crucial for PM-induced immunotoxicity (86). PM exposure and NLRP3 inflammasome activation have been connected, but the underlying mechanisms remain unknown (85, 87). However, there may be a connection between PM exposure and reduced intracellular ATP levels (85, 86, 87). Additionally, extracellular ATP has been shown to be an NLRP3 inflammasome activator (87). Thus, it is likely that ATP mediates the link between PM exposure and the NLRP3 inflammasome (85, 87, 88).
PM2.5-induced pro-inflammatory cytokine production
AhR activation, ROS production, and p38 MAPK phosphorylation are the mechanisms by which PM induces inflammatory responses in HaCaT cells through inflammatory cytokine secretion, namely IL-1α, IL-1β, IL-6, and IL-8 (89). Using recombinant versions of these interleukins, it was shown that keratinocyte-released IL-1α and IL-1β increased MMP1 and COX2 expression in human dermal fibroblasts following PM exposure (89). This PM-induced IL-1α and IL-1β release triggers dermal collagen degradation via a p38 kinase-dependent mechanism in fibroblasts (89, 90). The fact that PM2.5 causes considerable epidermal thickening and neutrophil infiltration in the dermis suggests that dermal inflammation is a result of the keratinocytic signaling pathway activation and generation of pro-inflammatory cytokines (83). Acute epidermal thickening and dermal inflammation seen in skin with damaged barriers raises the possibility that PM2.5 provokes an inflammatory reaction, leading to more skin damage (91). Repeated PM2.5 application can cause severe dermal inflammation with mostly neutrophil infiltration, increasing the severity of inflammatory skin diseases (84, 91).
Go to : 
CONCLUSION
PM is a key indicator of air pollution caused by both natural and anthropogenic activities. As it spreads in the environment over time, it can induce a range of illnesses, resulting in increased mortality and a decrease in quality of life. Thus, PM can play a substantial role in the onset and progression of inflammatory skin conditions. PM causes skin barrier dysfunction by activating different inflammatory cascades, unbalancing T cell differentiation, triggering NLRP3 inflammasome activation, and inducing pro-inflammatory cytokine production. However, future research should focus on mechanistic investigations by using cell-based and animal models to understand how PM adversely affects skin’s immune system. This endeavour will demand for solving human health issues by PM exposure. Overall, this review will broaden our knowledge and help develop better strategies to manage human health challenges associated with PM.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download