INTRODUCTION
Equine infectious respiratory disease is one of the most common causes of lost training days and reduced performance in racehorses, leading to significant financial cost to the equine industry
(1,
2). EIV, EHV-1, EHV-4, ERAV, ERBV, and
S. equi have been well recognized as causal agents of equine infectious respiratory disease
(1,
2,
3). EIV of the H3N8 subtype has particularly been considered as the most important respiratory pathogen of horses due to its remarkably contagious nature and ability to spread rapidly through horse populations
(4). In contrast, H7N7 viruses have not been isolated for over 20 years and are regarded as extinct
(4). Both EHV-1 and EHV-4 are prevalent ubiquitously in horse populations throughout the world and most horses have serological evidence of exposure to these viruses
(5).
EHV-1 and EHV-4 can cause respiratory infections characterized by nasal discharge and pyrexia
(5). Mild or subclinical infections are common for both viruses
(6). Horses are repeatedly infected by both viruses naturally and these viruses can establish life-long latency
(6,
7). Transmission can occur through contact with nasal discharge, direct horse to horse contact, aerosol contamination and contact with contaminated feed or equipment
(6). ERAV and ERBV are also prevalent worldwide and seropositivity rates in adult horses can approach 100%
(8). ERAV and ERBV infection can result in pyrexia, serous nasal discharge, pharyngitis, bronchitis, coughing and swollen lymph nodes
(8). However, the severity and frequency of these signs vary and most infections appear to be subclinical
(8). Common transmission of both viruses is thought be through contact with nasal secretions and aerosols
(8).
Streptococcus equi subspecies
equi, the causal agent of strangles, is frequently diagnosed among equine populations worldwide
(9,
10). This infection is characterized by enlarged and abscessated mandibular and retropharyngeal lymph nodes
(11). Recovered horses can be carriers of
S. equi, which persistently shed and transmit the organism, without showing obvious clinical signs
(10,
11). Subclinical infections as causes of reduced performance in athletic horses have been reported previously
(12). Equine infectious respiratory agents can exist in horses without causing obvious clinical signs and so infections can pass unnoticed
(8,
10). The absence of EIV and detection of EHV-1, EHV-4, ERAV, ERBV, and
S. equi using PCR assay in Thoroughbred racehorses in Republic of Korea have been reported
(1,
13).
Outbreaks of respiratory disease occur on premise where horses congregate such as racetracks
(14). At the SRP, approximately 1,301 Thoroughbred race horses are trained
(15) and 865 race games are conducted annually
(16). The racehorses are raised and trained closely together at the SRP by sharing of horse management facilities such as horse walkers, swimming pools, and tracks. EIV, EHV-1, EHV-4, ERAV, ERBV, and
S. equi can be transmitted by direct horse-to-horse contact and aerosolized droplets of nasal secretions
(17,
18,
19). In particular, outbreaks of EIV, EHV-1, and EHV-4 can cause severe clinical and economic damage to the horse racing industry
(20). Therefore, surveillance of these respiratory infectious agents is important for estimating the possible risks and preventing outbreaks of respiratory infectious diseases among racehorses at the SRP.
However, to the authors’ knowledge, serological surveillance has not been conducted in racehorses in Republic of Korea. The aim of this study was to determine the post-vaccination antibody levels to EIV (H3N8), to identify the seroprevalence of EHV-1, EHV-4, ERAV, ERBV and S. equi in Thoroughbred racehorses in the SRP and to make recommendations for regular testing in order to prevent possible decreased performance, health status and financial loss.
RESULTS
Table 1 summarizes the number of horses with different levels of EIV antibody (<50, 50-85, 85-150 and >150 mm
2). Seventy eight out of 94 samples (83.0%) had haemolytic areas over 85 mm
2, which was interpreted as clinically protected against EIV infection. The results of serological tests for EHV-1, EHV-4, ERAV, ERBV and
S. equi are presented in
Table 2.
Table 1.
Equine influenza H3N8 single radial haemolysis (SRH) levels in SRH Thoroughbred race horses
|
SRH
|
Number of horses
|
Mean (mm2)
|
Median (mm2)
|
|
≤50 mm2
|
3 (3.2%)
|
30.5
|
44.4
|
|
>50 mm2 and ≤85 mm2
|
13 (13.8%)
|
73.6
|
76.2
|
|
>85 mm2 and ≤150 mm2
|
65 (69.2%)
|
123.0
|
123.6
|
|
>150 mm2
|
13 (13.8%)
|
173.3
|
167.2
|
Table 2.
Serology results for respiratory agents in Thoroughbred racehorses in Republic of Korea
|
Agent
|
Test (cut off)
|
Positive (n)
|
Negative (n)
|
Prevalence (%)
|
|
EHV-1
|
Complement fixation (≥1:80)
|
9
|
85
|
9.6
|
|
EHV-4
|
Complement fixation (≥1:80)
|
29
|
65
|
30.9
|
|
ERAV
|
Complement fixation (≥1:80)
|
1
|
93
|
1.1
|
|
ERBV
|
Complement fixation (≥1:80)
|
1
|
93
|
1.1
|
|
S. equi
|
ELISA (≥0.5)
|
2
|
92
|
2.1
|
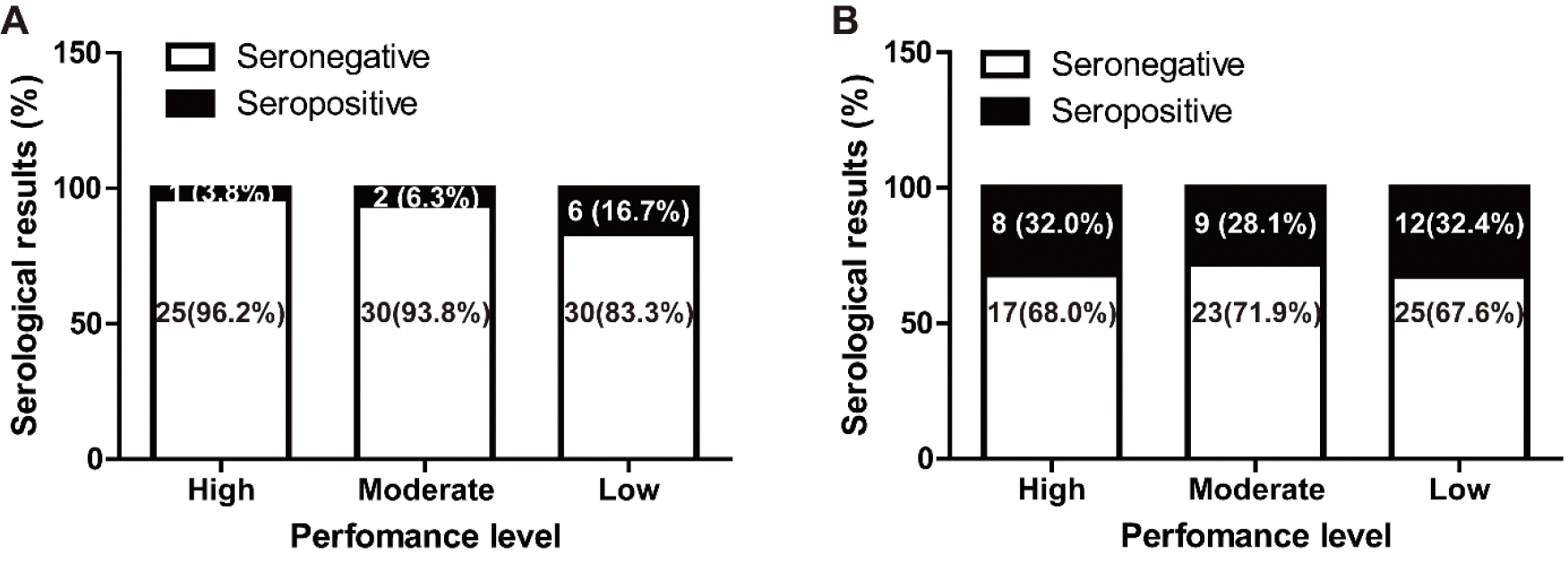
Horses with antibodies to EHV-4 were the most prevalent (30.9%), followed by EHV-1 (9.6%). Six horses were seropositive for both EHV-1 and the EHV-4 CF test (6.4%). However, the prevalence of
S. equi-, ERAV-, and ERBV-positive horses were too low in this study and the race results were not investigated for those pathogens. The race results were investigated for EHV-1 and EHV-4 positive horses. The SPSS cross table summarizes the relationship between race results and antibodies against EHV-1 and EHV-4 (
Table 3). High and moderate performance level in EHV-1 seropositive horses was 33.3% (3 out of 9) and 64.7% (55 out of 85) in the EHV-1 seronegative group. High and moderate performance level was observed for 62.1% (18 out of 29) of the EHV-4 seropositive group and 61.5% (40 out of 65) of the EHV-4 seronegative group. In each case, EHV-1 was not significantly linked to performance level (
p = 0.081, Fisher’s exact two-sided test). And, EHV-4 was not linked to performance level (
p = 0.789, Pearson’s chi-squared test). As shown
Fig. 1, it seems to have a negative correlation between the race results and antibodies against EHV-1 (p = 0.080, linear by linear association), however, the result was not statistically significant (p > 0.05). And, in the EHV-4 case, no significant correlation was measured (
p = 0.765, linear by linear association).
Table 3.
Performance level between seropositive and negative groups for EHV-1 and EHV-4 in Thoroughbred race horses in Republic of Korea (n=94)
|
Performance level
|
EHV-1
|
EHV-4
|
|
Positive (%)
|
Negative (%)
|
Positive (%)
|
Negative (%)
|
|
Higha and Moderateb
|
3 (33.3%)
|
55 (64.7%)
|
18 (62.1%)
|
40 (61.5%)
|
|
Higha,d
|
1 (11.1%)
|
25 (29.4%)
|
9 (31.0%)
|
17 (26.1%)
|
|
Moderateb,e
|
2 (22.2%)
|
30 (35.3%)
|
9 (31.0%)
|
23 (35.4%)
|
|
Lowc,f
|
6 (66.7%)
|
30 (35.3%)
|
11 (37.9%)
|
25 (38.5%)
|
|
Total
|
9 (100%)
|
85 (100%)
|
29 (100%)
|
65 (100%)
|
Fig. 1
Performance level between seropositive and negative groups for EHV-1 (A) and EHV-4 (B) in Thoroughbred race horses in Republic of Korea (n=94).
DISCUSSION
Infectious respiratory diseases are important to the training and performance of racehorses
(9). In addition to affecting an individual’s athletic performance, outbreaks of acute infectious respiratory disease can lead to the cancellation of race events with huge financial consequences
(2). EIV, EHV-1, EHV-4, ERAV, ERBV, and
S. equi are important and common infectious respiratory pathogens and can be transmitted by nasal secretion, aerosol and direct contact
(17,
19). Racehorses typically have an increased likelihood of developing respiratory infections due to their: relatively young age lacking a fully developed immunity to infectious agents, stabling in large groups, which is favorable for the introduction and exposure to infectious agents and the sharing of facilities in training with increased exposure via aerosols and contaminated surfaces
(2). Approximately 1,369 Thoroughbred racing horses at the SRP lived together and had training on a communal race track. Most of the horses at the SRP are a relatively young with an average age of 3.4 years old. Thus, our data suggest that the athletic horses at the SRP are at risk of outbreak or endemic transmission of respiratory pathogens. In 2008, 3 of 72 Thoroughbred racehorses with respiratory disease at the SRP were positive for
S. equi by bacterial culture
(13). The absence of EIV and existence of EHV-1, EHV-4, ERAV, and ERBV from nasal swabs and serum samples in the SRP has been reported using molecular detection methods in 2013
(1). To the authors’ knowledge, however, few studies conducting serological surveillance for these infectious pathogens have been conducted.
The SRH test has proven to be useful in the serological diagnosis of immunity to EIV infection
(4). In the SRH test, the size of haemolytic zone is directly proportional to the level of strain-specific antibody in the serum samples, which correlated closely with protective immunity
(23). In this study, the mean SRH level of 120 mm
2 was similar to the level of 121 mm
2 and 115 mm
2 reported for Thoroughbred horses in training in Ireland and the UK respectively
(22,
23). Comparing with the previous result of Thoroughbred training horses in Ireland
(22), the horses in this study with antibody levels of 85 mm
2 or greater, which was interpreted as clinically protected against EIV, was the same at 83.0%. The antibody levels of partially protected horses against EIV was 13.8% (13 out of 94) in this study, while the SRH of Thoroughbred training horses in Ireland was 9.0% (20 out of 223). Like EIV vaccination regulation for Thoroughbred training horses in Ireland
(22), all Thoroughbred horses in the SRP are required to be vaccinated at 6 month intervals. Therefore, it is thought that the protective immunity against EIV in Thoroughbred racehorses at the SRP is as high as in other populations of Thoroughbred performance horses.
The CF test, which detects the immunoglobulin M (IgM) antibody for EHV-1 and EHV-4 is also an internationally recognized test and can be used to identify acute infection to both viruses
(24). EHV infection induces a short-lived (<3 months) IgM response, followed by a longer-lived (>12 months) IgG response. The CF test is used to measure the short-lived (IgM) antibody response in diagnostic laboratories
(24). CF titers increase instantly after infection, peak rapidly, and then decrease
(24). Therefore, a high CF titre in a single serum sample suggests evidence of infection and can be used as initial diagnostic test in EHV cases
(24). Seropositivity to EHV-1 and EHV-4 was more common in this study, which is consistent with a previous report in young racehorses
(25). The performance levels of EHV-1 and EHV-4 positive and negative groups were compared. There was no significant difference between each EHV-1 and performance levels, and EHV-4 and performance levels. However, there was a trend for EHV-1 to be closely related than EHV-4 (
p = 0.081 versus
p = 0.789). The proportion of horses with high and moderate performance levels in the EHV-1 seropositive group (33.3%) was almost significantly less than in the EHV-1 seronegative group (64.7%). The proportion of high and moderate performance levels in the EHV-4 seropositive group was 62.1% versus 61.5% in the EHV-4 seronegative group. Although there are no universally accepted standard definition for poor performance, and noting its multifactorial causes
(26), the race result of EHV-1 seropositive group was quite distinctive in the study. EHV-1 infection can be reactivated in horses with latent infection and stress factors such as strenuous physical training, transportation, and fatigue can induce the virus pathogenicity
(27). EHV-1 infected horses can have subclinical signs of minimal severity
(17), and some horses recovered from EHV-1 respiratory infection may develop a poor performance syndrome
(17). High virulence strains of EHV-1 and -4 cause more severe clinical signs
(17). In our study, EHV-4 seropositivity was similar in the high, moderate, and low performance groups (31.0%, 31.0%, and 37.9%, respectively). Further, the racehorses at the SRP were considered to be infected with the less virulent EHV-4 strains, and EHV-4 seropositivity did not affect their performance. Our results suggest that subclinical EHV-1 infection may have affected the athletic performance reduction in the racehorses in this study. However, sample size was limited (n = 94), and each race had different race level in this study. Therefore, a larger study is now required to confirm this trend.
In a previous study by Black
et al., the seroprevalence of ERAV and ERBV in Thoroughbreds in training were relatively high (61% and 77%–86%, respectively)
(28). The seroprevalence of
S. equi in racehorses was also high (45.7%) in a study conducted in 2017
(29). However, the seropositivity of ERAV, ERBV, and
S. equi in Thoroughbred racehorses at the SRP was much lower than that reported in previous studies (1.1%, 1.1%, and 2.1%, respectively). The molecular prevalence of ERAV and ERBV was as low as 2.2% and 6.7%, respectively, in racehorses in Republic of Korea in 2013
(1). Although ERAV and ERBV were present, only a small number of racehorses were infected at the SRP. There is a difference in the test method for
S. equi. Moghaddam
et al. reported a seroprevalence of 45.7% in racehorses during 2017 using an iELISA based on SeM, which is an M-like fibrinogen-binding surface protein of
S. equi (29). However, iELISAs using the full SeM protein have been criticised for their inferior specificity due to cross-reactivity with
Streptococcus zooepidemicus (18,
30). In 2013, Robinson
et al. developed a dual antigen A (N-terminal fragment of SEQ2190) and C (N-terminal fragment of SeM) iELISA kit, which is beneficial in terms of much lower cross-reactivity with
S. zooepidemicus (99.3% specificity) and high sensitivity (93.3%)
(10,
11,
18). Thus the duplex iELISA kit incorporating SEQ2190 and SeM was used in this study. The difference in cross-reactivity in detecting
S. equi using the study method was affected by the lower seropositivity result compared to that reported in a previous study (2.1% versus 45.7%).
In addition, racehorses at the SRP are a closed population and most horses are raised, trained, and raced at the SRP. Biosecurity protocols are performed at the SRP to prevent airborne infectious agents. Disinfection of all the stables and the environment is implemented regularly twice a week. Regular vaccination programs are conducted for all racehorses at the SRP. When entering the SRP, only vaccinated and clinical health certified horses are allowed, while newly imported horses are quarantined and inspected for respiratory clinical signs by veterinary staffs. Therefore, it is thought that the infestation potential of ERAV and S. equi is low due to the closed population with a rigid biosecurity system at the SRP.
Using CF tests and dual antigen ELISA kit for S. equi, the recent infection or carrier status of EHV-1, EHV-4, ERAV, ERBV, and S. equi in Thoroughbred racing horses in the SRP have been revealed in this study. High levels of antibodies against EIV linked to regular vaccination was determined through SRH test. Although many racehorses are raised in the SRP and kept in close contact with each other, overall seroprevalence of important respiratory pathogens was relatively low, providing evidence of low risk of respiratory infectious agents in Thoroughbred racehorses in the SRP. EHV-4 was the most prevalent pathogen, with 30.9% seropositive horses, and both ERAV and ERBV were the least prevalent at 1.1% in this study. A trend towards reduced performance was found in EHV-1 seropositive racehorses in this study. Further studies are required to confirm the relationship between the EHV-1 infection and sub-optimal athletic performance in racehorses.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was declared.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download