INTRODUCTION
Enterovirus 71 (EV71) is a single positive-strand RNA virus belonging to the enterovirus genus within the family Picornaviridae (1). EV71 infection occurs throughout the year but is most common in summer and autumn (2, 3). It is one of the primary pathogens of hand-foot-mouth disease (HFMD) in young children and infants. HFMD caused by EV71, but not by other enteroviruses, is sometimes involved with severe neurological diseases such as aseptic meningitis, brain stem encephalitis, poliomyelitis-like paralysis, and death, accounting for 80% of severe and 93% of fatal HFMD cases (4, 5, 6, 7). EV71 consists of a single open reading frame flanked by 5' and 3' untranslated regions (UTRs). The 5'-UTR includes an internal ribosome entry site (IRES), which initiates the synthesis of the viral polyproteins (VP1-VP4) and non-structural proteins (2A-2C and 3A-3D) (8). All enteroviruses express two separate proteases, 2A and 3C, which are used to usurp infected host cell functions. Each protease targets different host cell substrates, as well as being responsible for processing different boundaries within the viral polyprotein. It is essential for cell proliferation and survival (9). The majority of antiviral drugs target a specific component of the virus. The high replication and mutation rates of enteroviruses can result in the virus developing resistance to this type of antiviral treatment (10). Targeting host factors such as cell signaling molecules and apoptosis inducers may establish a higher genetic barrier to resistance and can be used in combination with viral inhibitors to treat these infections (10). Previous reports demonstrate that the regulation of ERK and Akt cell signaling activity is important to the propagation of CVB3 in infected cells. The inhibition of both signalings were completely blocked CVB3 replication in HeLa cells (11, 12). Especially, Akt signaling inhibition by using PI3K inhibitor strongly suppressed CVB3 propagation and subacute phase myocardium damage in the myocarditis mouse model (13). However, to date, there is no effective treatment for enteroviruses and there are high rates of disability and mortality due to the lack of effective preventive and treatment drugs.
Streptomyces are commonly reported as soil bacteria with a lifestyle similar to fungi. Streptomyces are gram-positive and have genomes with high GC content. Previously reported that this strain produced highly effective antibiotics against multidrug-resistant Acinetobacter baumannii (14, 15). However, the antiviral effects of Streptomyces extract are never studied before.
In this study, we screened several ocean organisms extracts and choose KH29 extract as a candidate for the anti-EV71 agent. KH29 extract inhibited EV71 replication, having a strong antiviral effect in HeLa cells. KH29 extract treatment activated the AKT signaling pathway, which promotes cell survival in EV71 infection. These results suggested that KH29 extract may be an effective therapeutic drug candidate for development as an anti-EV71 treatment.
Go to : 
MATERIALS AND METHODS
Cell line and virus
EV71 (Enterovirus A71 stain, gift from Korea National Institute of Health) was cultured on HeLa cell monolayers. HeLa cells were grown for 16 h and infected with 107 plaque-forming units (PFU) of EV71. When the cytopathic effect (CPE) of the infected cells reached > 90%, the cells were subjected to three freeze-thaw cycles at -80℃. Virus stock concentrations were determined by tissue culture infectious dose 50 (TCID50). HeLa cells were cultured using Dulbecco’s modified eagle medium (DMEM, Welgene, Inc., Gyeongsan-si, Korea) with 5% fetal bovine serum (FBS), 1% penicillin-streptomycin sol. (Welgene, Inc) at 37℃ in a humidified 5% CO2 incubator (16).
In vitro antiviral effect screening
We have screened the antiviral activity of various chemicals and organisms extract by in vitro cell survival assay. All samples (emetine, hypericin, tanshinone, Streptomyces microflavus extract, Streptomyces anulatus extract, and Streptomyces sp extract) were provided by professor Sang-Jip Nam from Ewha Womans University (Seoul, Korea). HeLa cells were cultured in 96-well-plate (3x104 cells/well). We treated organism extract that was serially diluted in DMEM (5% FBS, 1% penicillin-streptomycin) in a dose-dependent manner (0.1-100 μg/㎖). EV71 (107 PFU/ml) was added to HeLa cells with or without extract and the positive control represents virus-only treatment well. After 19 hrs of incubation, the cell was applied for 3-(4,5-Dimethyl –2- thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. Briefly, the culture medium was changed to 100 ㎕ serum-free medium. Then 5 ㎎/㎖ of MTT solution was added to each well. The culture medium was removed after 4 hrs and the MTT crystal was dissolved with 100 ㎕ DMSO. Cell survival was measured by a microplate reader at 575 nm wavelength after MTT solution treatment.
Virus infection and KH29 extract treatment
HeLa cells were cultured in 24well-plate (5x104 cells/well) for 18 hrs before the experiment. The cells were infected by EV71 (107 PFU/ml) with KH29 extract which was serially diluted in a culture medium (0.1-100 μg/㎖). After 8 hrs of incubation, when the cell CPE was observed the total protein or RNA was extracted by phosphate-buffered saline (PBS) lysis buffer (1x PBS, 0.5 mM EDTA, 0.1% Triton X-100, and Protease inhibitor cocktail) or TRIzol reagent (ThermoFisher Scientific, Cambridge, MA, USA). The protein and RNA were applied for western blot analysis or reverse transcription (RT)-PCR (17). Akt was inhibited by 50 mg/ml PI3K inhibitor (LY294002: Cell Signaling, Danvers, MA, USA) treatment.
Western blot analysis
Protein was extracted using PBS lysis buffer (1x PBS, 0.5 mM EDTA, 0.1% Triton X-100, and Protease inhibitor cocktail). The cell lysate was centrifuged at 4℃, 13000 rpm, for 5 minutes. Supernatant aliquots of total cell extracts were loaded onto 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) gels. After electrophoresis, the cells were transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked in 5% non-fat dry milk solution in tris-buffered saline containing 0.1% Tween 20 (TBST) and probed with antibodies against EV71-VP1 (VP1, 1:1000Merck Millipore, Darmstadt, Germany), eukaryotic translation initiation factor 4 Gamma-1 (eIF4G1, 1:1000), glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:2500), phospho-protein kinase B (Akt Ser473, 1:2500), phospho-p38 mitogen-activated protein kinase (MAPK Thr180/Tyr182), phospho-glycogen synthase kinase 3β (GSK-3β Ser9), and phospho-nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB p65, 1:1000)(Cell Signaling) for overnight at 4℃ and then membrane was incubated with HRP-conjugated secondary antibodies. The protein band was detected by the Chemi-doc system (Bio-Rad, Hercules, CA, USA) after ECL solution treatment (17).
Immunofluorescent stain
EV71 proliferation was confirmed by the immunofluorescent stain of viral capsid protein EV71-VP1. HeLa cells were infected by EV71 with serial-diluted KH29 extract. After 16 hrs, HeLa cells were fixed by cold methanol for 15 min, followed by blocking and permeabilization with 2% Bovine serum albumin (BSA) and 0.2% Triton X-100 in PBS and incubated with primary antibodies of rabbit EV71-VP1 antibody (Thermo Fisher Scientific). Target proteins were visualized with secondary antibodies conjugated with fluorophores (Alexafluor 488, 1:250; Thermo Fisher Scientific) and Hoechst nuclear stain. Fluorescence images were taken and processed using a fluorescent microscope (Olympus Co., San Jose, CA, USA).
Reverse transcription PCR
The viral RNA (positive or negative strand) genome amplification was observed by reverse transcription (RT)-PCR, and total RNA was isolated from EV71-infected and non-infected HeLa cells by TRIzol reagent (ThermoFisher Scientific). The RT reaction was performed using the Maxime RT kit (Intron Biotech, Inc., Seongnam-si, Korea) with 2 µg of total RNA as the template. PCR with the synthesized cDNA was performed with primers of EV71 Sense 5'-GTTCTTAACTCACATAGCA-3', and EV71 VP1 Anti-sense 5'-TTGACAAAAACTGAGGGGTT-3' GAPDH Sense 5'-ATCAACGACCCCTTCATTGAC-3', and GAPDH Anti-sense 5'-CCAGTAGACTCCACGACATACTCAGC-3' with cDNA as template. Then, the PCR product was electrophoresed on 1.5% agarose gel, and viral VP1 gene positive and negative strands were quantified by semi-quantitative RT-PCR. All data were quantified by NIH-image J V1.45 software and normalized by GAPDH as described previously (16).
Statistics
The data are presented as the mean ± SEM. The results of the control and virus infection groups were analyzed by Student t-test by Prism 3.0. Statistically, * p <0.05, ** p <0.01, *** p <0.001 was considered significant.
Go to : 
RESULT
Antiviral effect screening
The extract antiviral effect was confirmed in EV71 infected HeLa cells. Efficacy was confirmed that 0.1~100 μg/㎖ of concentration with EV71 infection (10). Among them, Streptomyces sp (KH29) extract showed a strong antiviral effect (Fig. 1A). The cytotoxicity was observed in HeLa cells by MTT assay (Fig. 1B). KH29 extract treatment was not shown any cytotoxicity in HeLa cells.
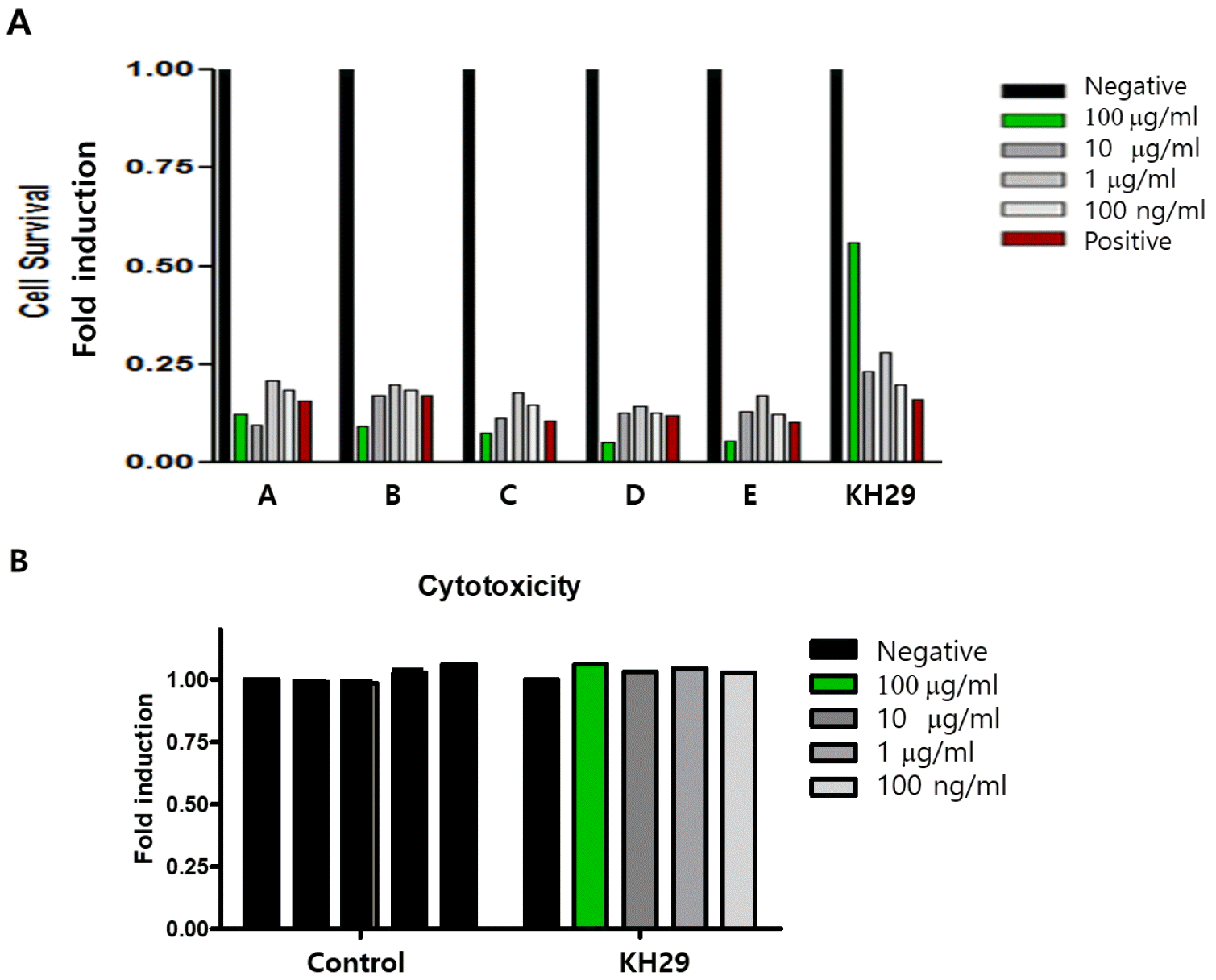 | Fig. 1Antiviral effect screening in EV71 infected HeLa cells. (A) The antiviral effect of the extract was screened by HeLa cell survival. EV71 antiviral effect was one time tested. (A: enetine, B: hypericin, C: tanshinone, D: Streptomyces microflavus, E: Streptomyces anulatus). (B) KH29 extract cytotoxicity was tested in HeLa cells. All data is from one experiment. Negative:no infection, positive: virus only. |
KH29 extract inhibits EV71 replication
We investigated the efficacy of KH29 extract to inhibit EV71 replication. In the early time of enterovirus infection, the virus produces proteases2A to cleave the EV71 polyprotein. However, the enteroviral protease2A cleaves the host protein eukaryotic translation initiation factor 4 gamma 1 (eIF4G1). As shown in western blot, viral capsid protein VP1 was dramatically decreased and cleavage of eIF4G1 was significantly decreased in 100 μg/㎖ of KH29 extract (Fig. 2). In addition, we measured EV71 replication by immunofluorescent staining of viral capsid protein VP1. EV71 infected HeLa cells were treated with 1, 10 μg/㎖ KH29 extract. EV71 VP1 was significantly decreased with 10 μg/㎖ of KH29 extract compared to untreated cells (Fig. 3). These results showed that KH29 extract efficiently inhibited EV71 replication.
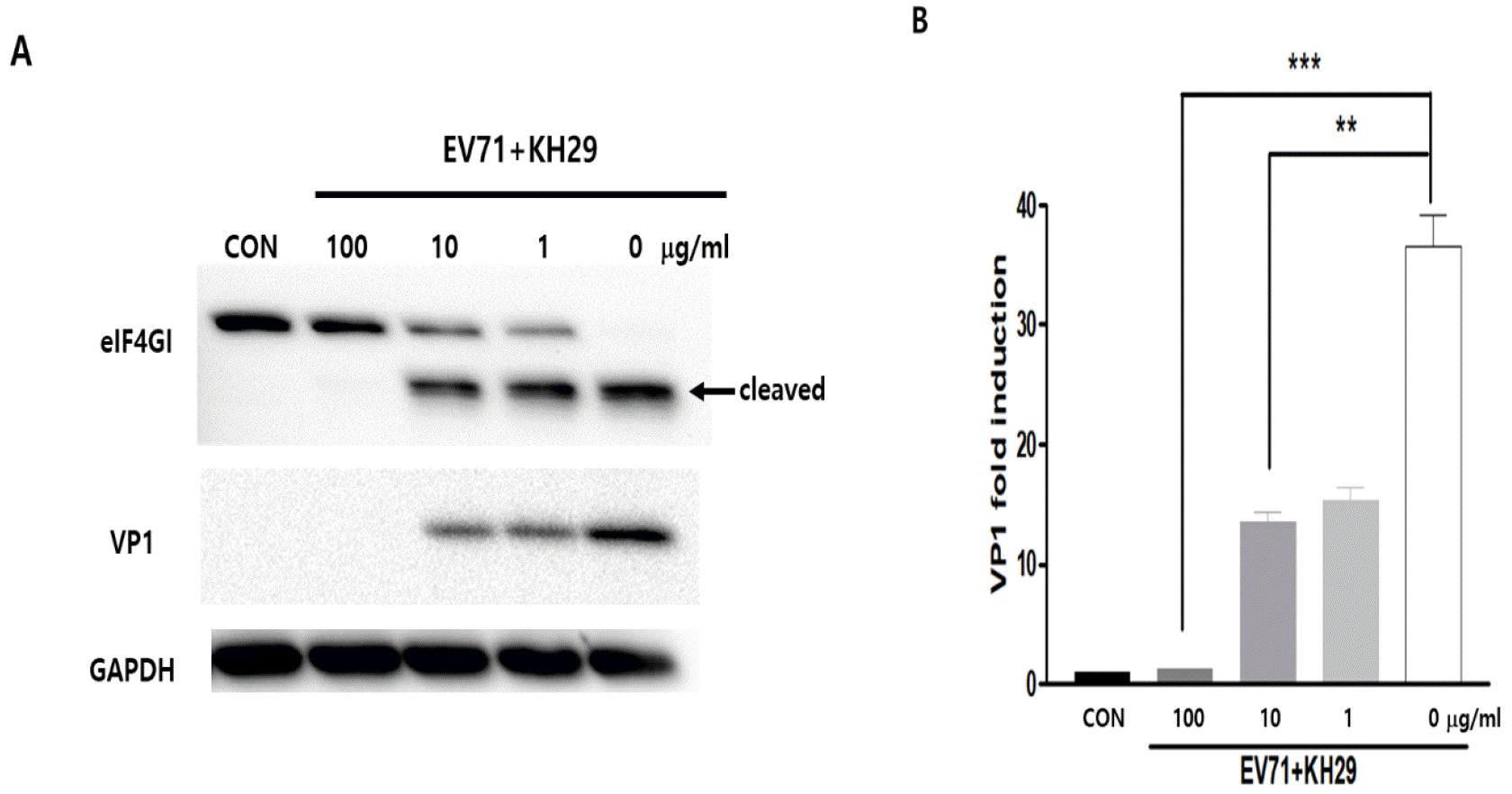 | Fig. 2KH29 inhibits EV71 infection and replication. (A) The effect of KH29 extract was confirmed by western blot analysis. KH29 treated HeLa cells protein was extracted and applied for western blot analysis. CON: no infection. (B) Western blot results were quantitated by NIH-ImageJ software. VP1 immunoblot bands indicate fold changes of VP1 normalized to GAPDH bands. All data are the mean ± s.e.m. from 2 independent experiments. **P<0.01 and ***P<0.001 by two-tailed Student’s t-test. |
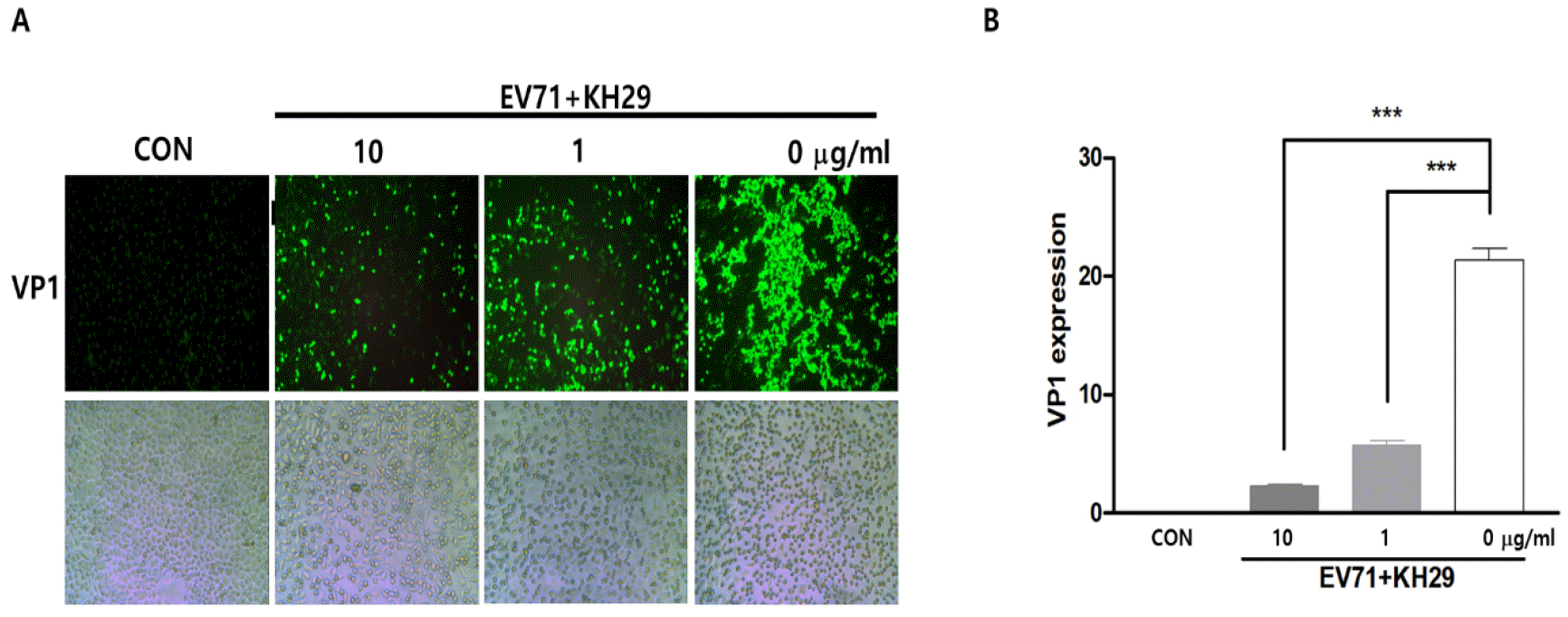 | Fig. 3KH29 significantly inhibited EV71 replication in HeLa cells. (A) Virus infected HeLa cell was applied for immunofluorescent stain subject to EV71 VP1 antibody. VP1 protein expression was shown as green (upper),. The light field showed the cell culture condition in the plate (bottom). 100x magnitude. (B) Immunofluorescent stain results were quantitated by NIH-Image J software and are presented as the mean ± s.e.m. ***P< 0.001. CON: no infection. |
KH29 extract inhibits EV71 gene amplification
Virus replication is directly followed by viral gene amplification. EV71 RNA genome replication was observed whether virus replication is directly affected by KH29 extract treatment or not, and viral VP1 genes positive- and negative-strand were quantified by semi-quantitative RT-PCR. KH29 extract treatment significantly inhibited positive- and negative-strand of viral gene amplification. These results suggested that KH29 extract was effective to inhibit early-stage of EV71 replication (Fig. 4).
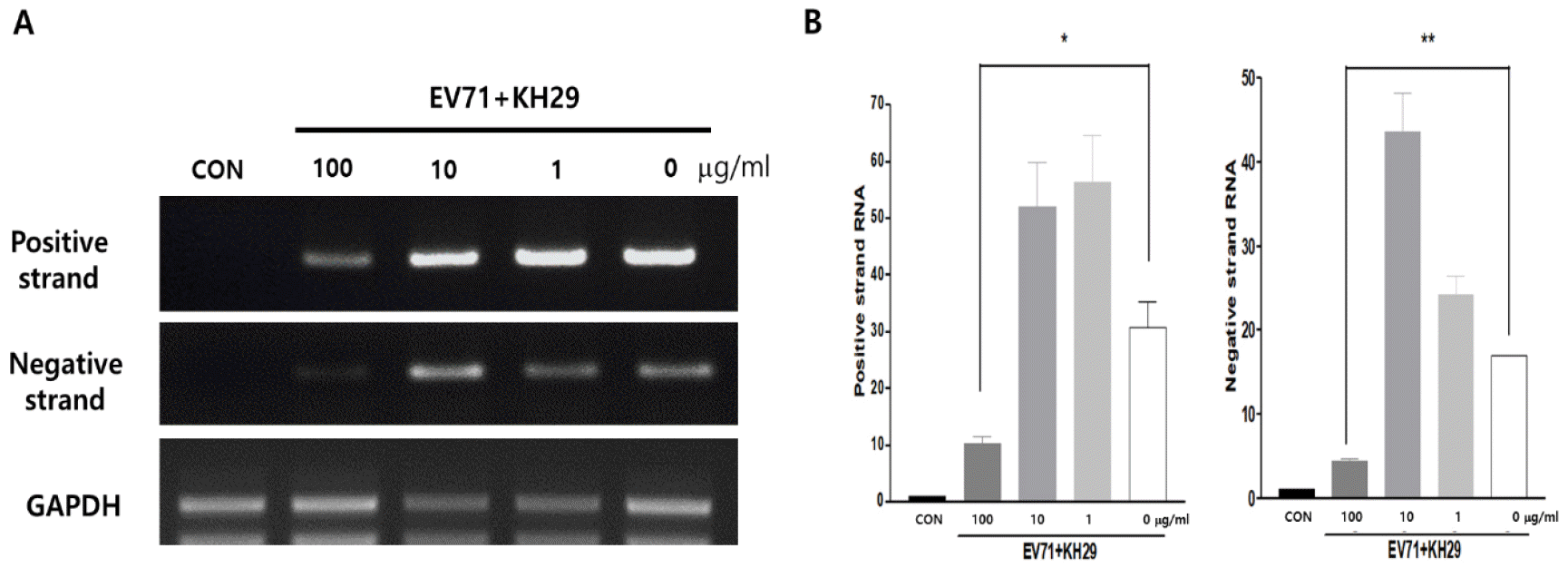 | Fig. 4KH29 extract inhibits EV71 gene amplification. (A) EV71 positive and negative gene numbers were quantified by RT-RCR. Positive-strand RNA was significantly reduced by 100 ug/ml of KH29 extract. Negative-strand RNA amplification was almost completely blocked. (B) Positive-strand RNA and Negative-strand RNA amplification results were quantitated by NIH-Image J software. CON: no infection. Positive and negative strand bands normalized to GAPDH bands. All data are the mean ± s.e.m. from 2 independent experiments. *P<0.05 and **P<0.01 by two-tailed Student’s t-test. |
KH29 extract activates Akt signaling
In previous studies, Enterovirus proliferation correlated with cell signaling regulation such as Akt activity at the late stage of infection (10). These findings suggested that this pathway may be important for EV71 proliferation after the initial infection. Therefore, KH29 inhibitory effects on EV71 replication may be through modulation of the Akt signaling pathway. To find out whether KH29 inhibitory effects on EV71 infection by Akt signaling activation, we treated 50 mg/ml of PI3K inhibitor (LY294002). Akt phosphorylation was significantly increased by 100 μg/㎖ of KH29 extract treatment compared to untreated in EV71 infected HeLa cells. In PI3K inhibitor treatment, p38 phosphorylation was increased by inhibition of Akt phosphorylation (Fig. 5). These results suggested that treatment of KH29 significantly increased cell survival through Akt activation in the early time of EV71 infection. Akt showed an opposite expression pattern with p38 activity.
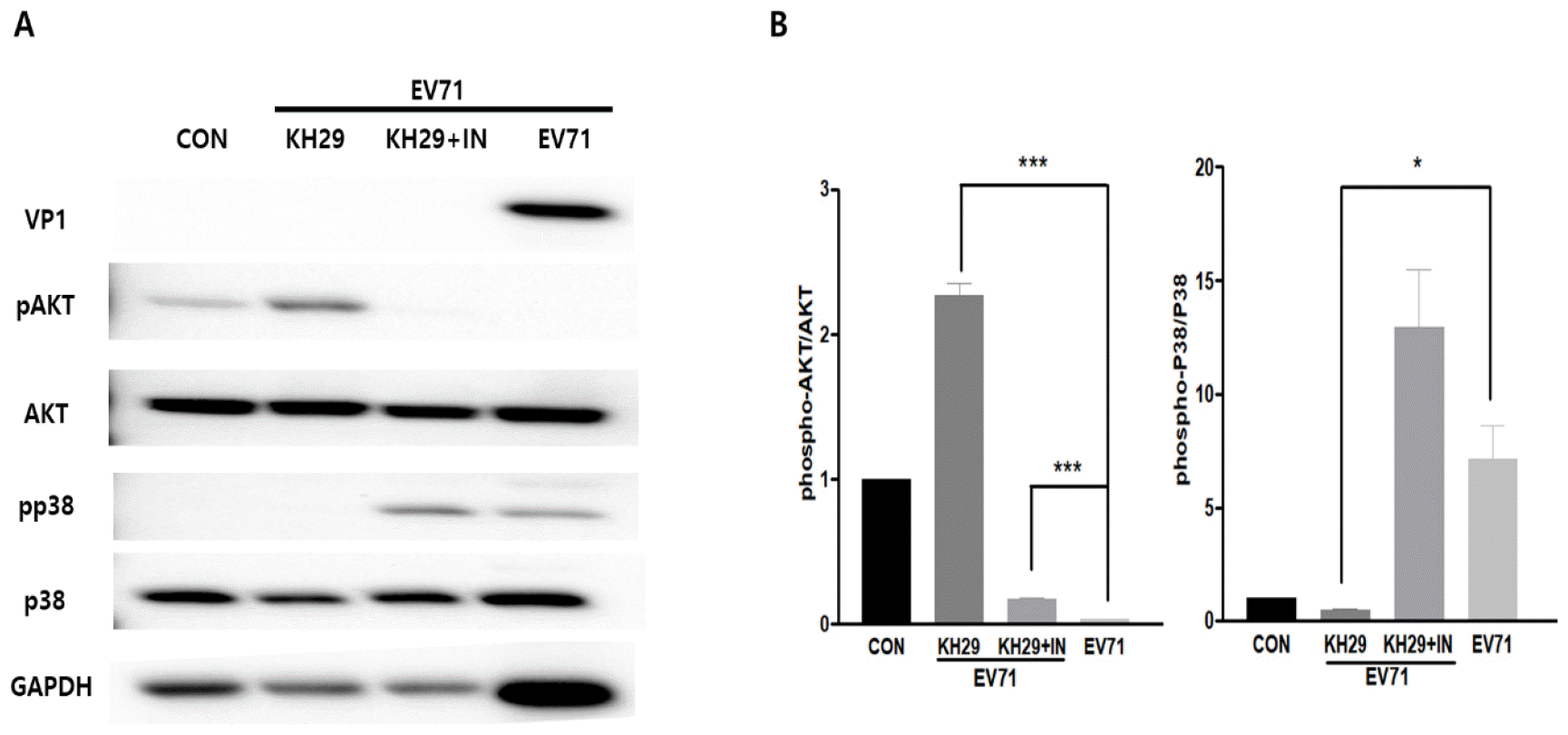 | Fig. 5KH29 extract activates Akt signaling in EV71 infection. (A) Akt signaling activity was significantly increased but p38 signaling was significantly decreased by KH29 extract (100 mg/ml). Akt signaling was blocked, but p38 signaling was increased by Akt inhibitor (50 mg/ml). (B) Western blot results were quantitated by NIH-image J software. pAkt and pp38 bands normalized to total Akt and p38 bands. CON: no infection. All data are the mean ± s.e.m. from 2 independent experiments. *P<0.05 and ***P<0.001 by two-tailed Student’s t-test. |
KH29 extract improves cell survival through GSK3β and NF-κB activation
EV71 replication is regulated by host cells signaling molecules such as GSK3β and NF-κB activity at the early stage of infection. Several well-characterized physiological substrates for Akt have been identified to date, including GSK-3 (11). GSK-3, a ubiquitously expressed protein–serine/threonine kinase, is inhibited by Akt phosphorylation in response to growth factor stimulation. These studies suggest that GSK3 is involved in multiple cellular processes, including metabolism, proliferation, and differentiation. We found that phosphorylation of GSK3β (Ser9) and NF-κB were significantly increased by KH29 extract treatment (Fig. 6). KH29 extract activates cell survival and proliferation through Akt signal-induced GSK3β (Ser9) and NF-κB phosphorylation during EV71 infection. Activated Akt phosphorylates and inactivates GSK3β which may improve cell survival in viral infection.
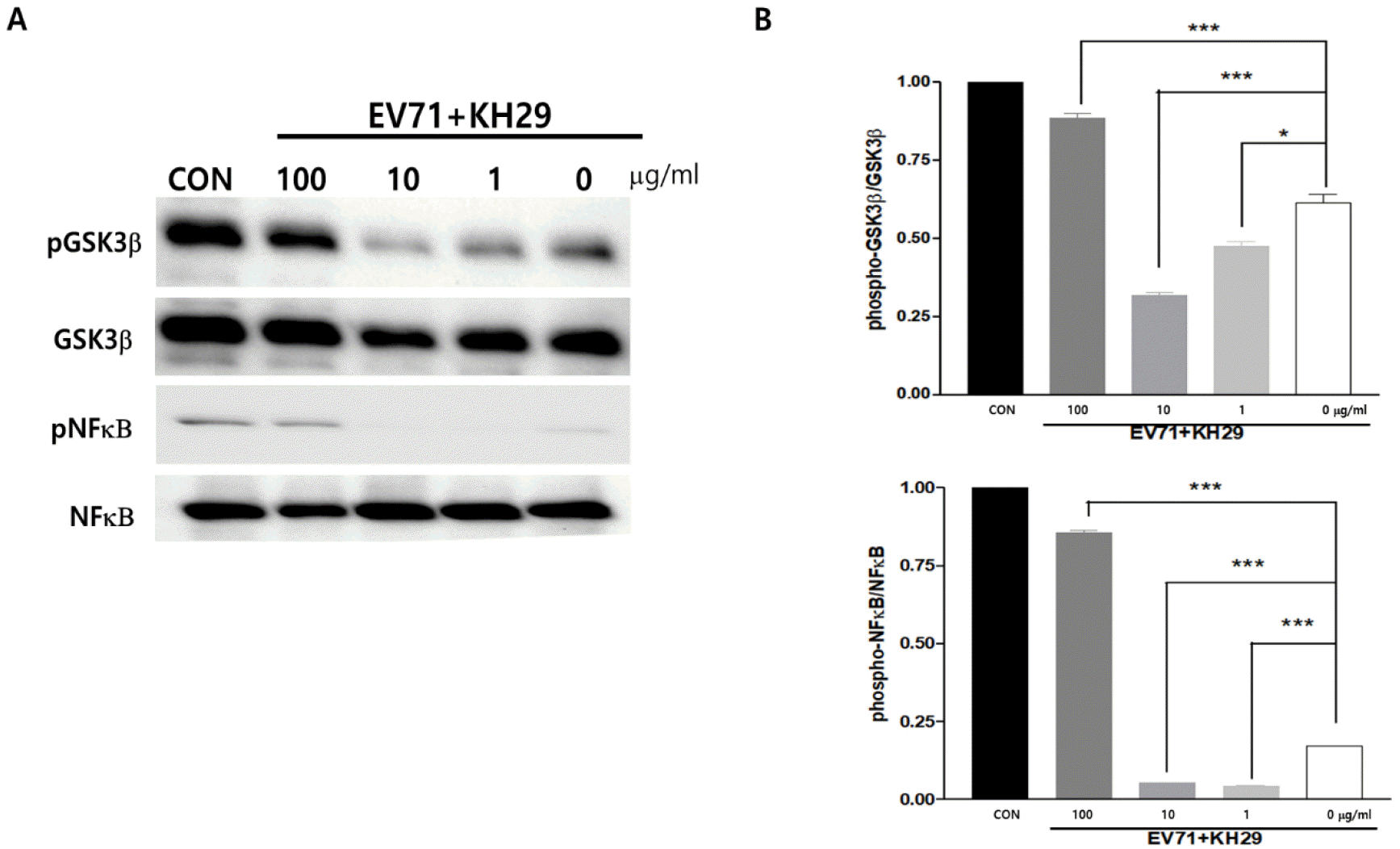 | Fig. 6KH29 extract regulates GSK3β and NFκB through Akt signaling pathway. (A) GSK3β (Ser9) and NFκB phosphorylation were significantly activated by 100 mg/ml of KH29 extract. (B) Western blot results were quantitated by NIH-image J software. pGSK3β and pNFκB bands normalized to total GSK3β and NFκB bands. CON: no infection. All data are the mean ± s.e.m. from 2 independent experiments. *P<0.05 and ***P<0.001 by two-tailed Student’s t-test. |
Go to : 
DISCUSSION
Enteroviruses are well known as a common cause of various human infectious diseases (18). In particular, EV71 belongs to the same Enterovirus genus and is the main causative agent of HFMD. It has been nearly 50 years since the first case of EV71 infection was reported in California in 1969, yet there is still no specific therapy for the severe inflammatory diseases, such as aseptic meningitis, that are occasionally caused by EV71. This virus has a small positive-strand RNA genome so can easily change outside capsid protein and generates a new variant virus. Some variants have more strong pathogenesis compared to the original one.
In this study, we found a new antiviral effect of KH29 extract against EV71, it is a strong activator of Akt that plays a vital role in regulating EV71 proliferation. KH29 was specifically selected from the ocean organisms extract due to its outstanding anti-EV71 ability. We observed that KH29 decreased EV71 capsid protein (VP1) expression. The cleavage of eIF4G1, a translation initiation factor that is cleaved by viral protease 2A (7, 8), was significantly decreased by KH29 treatment compared to untreated control. In immunofluorescent staining, cytoplasm viral protein (VP1) direct deposition was significantly decreased by 10 ug/ml of KH29 extract treatment compared to untreated control. Moreover, viral RNA genome amplification was significantly inhibited by KH29 extract.
Previous studies have confirmed that phosphorylation of ERK1/2 and Akt can regulate EV71 proliferation (10, 11, 13). EV71 replication is regulated by host cells signaling molecules such as GSK3β and NF-κB activity at the early stage of infection. The virus alters cell signaling and can affect cell survival and viral replication. Therefore, we hope that activation or suppression of cell signaling molecules will be an important target for the development of new antiviral drugs. We have confirmed that Akt active substances also have a strong antiviral effect. Akt inhibitor treatment significantly increased p38 phosphorylation in EV71 infection, even when using KH29 extract. However, EV71 proliferation was completely blocked by Akt inhibition. While this part is still a bit confusing, activation of Akt signaling by KH29 extract treatment inhibited p38 phosphorylation and viral replication of the data. The debate between inhibitory and activating effects at various points in EV71 infection and proliferation needs to be studied more. This is because the virus relies on utilizing the survival of the host cell for the amplification of its genes and offspring. In addition, GSK3β and NF-κB, which regulate EV71 replication, were dramatically induced by KH29 extraction treatment. Therefore, KH29 extract is predicted to inhibit viral replication through activation of the Akt pathway rather than the p38 pathway. The KH29 extract can be adjusted to activate Akt and increase the GSK3β signal when Akt is enabled. Therefore, cell proliferation and survival will be improved through NF-kB transcriptional regulation.
In this study, we found that KH29 extract activates Akt signaling in EV71 infected Hela cells and inhibits EV71 replication. Thus, KH29 can inhibit viral replication via activation of the Akt signaling pathway and downstream GSK3β and NF-kB molecule activation. These results suggest that KH29 extract can be a useful compound for the development of new therapeutic agents for the treatment of HFMD.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download