INTRODUCTION
Table 1.
| Study | No. of patients | Design | Timing of randomization | Treatment groups | Primary endpoint | Key findings |
|---|---|---|---|---|---|---|
| TOPIC [8] | 645 | Single-center, open-label, randomized trial in ACS patients | 1 mo post-ACS | Switched DAPT (from prasugrel or ticagrelor to clopidogrel) vs. continued DAPT with prasugrel or ticagrelor | Composite of cardiovascular death, urgent revascularization, stroke, and BARC ≥2 bleeding at 1 yr | Primary endpoint: 13.4% in the switched DAPT group vs. 26.3% in the unchanged DAPT (HR, 0.48; 95% CI, 0.34–0.68; P<0.01) |
| HOST-REDUCE-POLYTECH-ACS [9] | 2,338 | Multicenter, open-label, randomized trial in ACS patients | 1 mo post-ACS | De-escalation (prasugrel 5 mg) vs. conventional (prasugrel 10 mg) | Composite of all-cause death, nonfatal MI, stent thrombosis, repeat revascularization, stroke, and BARC ≥2 bleeding at 1 yr | Primary endpoint: 7.2% in the de-escalation group vs. 10.1% in the conventional group (HR, 0.70; 95% CI, 0.52–0.92; P=0.012) |
| TALOS-AMI [10] | 2,697 | Multicenter, open-label, randomized trial in AMI patients | 1 mo post-AMI | De-escalation (from ticagrelor to clopidogrel) vs. active control (continued ticagrelor) | Composite of cardiovascular death, MI, stroke, or BARC 2, 3, or 5 bleeding at 1 yr post-AMI | Primary endpoint: 4.6% in the de-escalation group and 8.2% in the active control group (HR, 0.55; 95% CI, 0.40–0.76; P=0.0001) |
| TROPICAL-ACS [11,12] | 2,610 | Multicenter, open-label, randomized trial in biomarker-positive ACS patients with successful PCI | 2 wk post-ACS | PFT guided de-escalation to clopidogrel vs. continued prasugrel | Composite of cardiovascular death, MI, stroke, and BARC ≥2 bleeding at 1 yr | Primary endpoint: 7% in the guided de-escalation group vs. 9% in the continued prasugrel group (HR, 0.81; 95% CI, 0.62–1.06; P=0.0004 for noninferiority; P=0.12 for superiority) |
| POPular Genetics [13] | 2,488 | Multicenter, open-label, randomized trial in STEMI patients undergoing primary PCI | 1–3 day after primary PCI | Genotype-guided therapy (ticagrelor or prasugrel in LOF alleles carriers and clopidogrel in noncarriers) vs. standard treatment with ticagrelor or prasugrel | Composite of death from any cause, MI, definite stent thrombosis, stroke, or PLATO major bleeding at 1 yr | Primary endpoint: 5.1% in the genotype-guided group vs. 5.9% in the standard-treatment group (HR, 0.87; 95% CI, 0.62–1.21; P<0.001 for noninferiority; P=0.40 for superiority) |
| PLATO major or minor bleeding at 1 yr | PLATO major or minor bleeding: 9.8% in the genotype-guided group vs. 12.5% in the standard-treatment group (HR, 0.78; 95% CI, 0.61–0.98; P=0.04) |
DAPT, dual antiplatelet therapy; ACS, acute coronary syndrome; TOPIC, Timing of Platelet Inhibition after Acute Coronary Syndrome; BARC, Bleeding Academic Research Consortium; HR, hazard ratio; CI, confidence interval; HOST-REDUCE-POLYTECH-ACS, Harmonizing Optimal Strategy for Treatment of Coronary Artery Diseases-Comparison of Reduction of Prasugrel Dose or Polymer Technology in Acute Coronary Syndrome Patients; MI, myocardial infarction; TALOS-AMI, Ticagrelor versus Clopidogrel in Stabilized Patients with Acute Myocardial Infarction; AMI, acute myocardial infarction; TROPICAL-ACS, Testing Responsiveness To Platelet Inhibition On Chronic Antiplatelet Treatment For Acute Coronary Syndromes; PCI, percutaneous coronary intervention; PFT, platelet function testing; POPular Genetics, CYP2C19 Genotype-Guided Antiplatelet Therapy in ST-Segment Elevation Myocardial Infarction Patients — Patient Outcome after Primary Percutaneous Coronary Intervention; STEMI, ST-segment elevation myocardial infarction; LOF, loss of function; PLATO, PLATelet inhibition and patient Outcomes.
DE-ESCALATION STRATEGIES
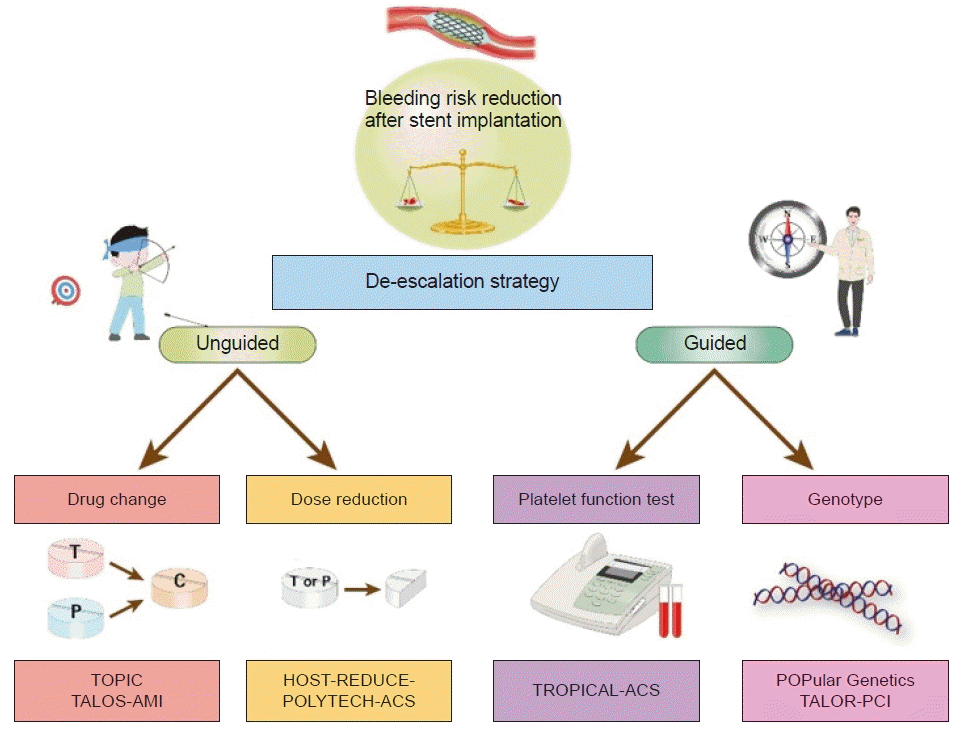 | Fig. 1.Different methods of de-escalation strategies of dual antiplatelet therapy in patients undergoing percutaneous coronary intervention for acute coronary syndrome. TOPIC, Timing of Platelet Inhibition after Acute Coronary Syndrome; TALOS-AMI, Ticagrelor versus Clopidogrel in Stabilized Patients with Acute Myocardial Infarction; HOST-REDUCE-POLYTECH-ACS, Harmonizing Optimal Strategy for Treatment of Coronary Artery Diseases-Comparison of Reduction of Prasugrel Dose or Polymer Technology in Acute Coronary Syndrome Patients; TROPICAL-ACS, Testing Responsiveness To Platelet Inhibition On Chronic Antiplatelet Treatment For Acute Coronary Syndromes; POPular Genetics, CYP2C19 Genotype-Guided Antiplatelet Therapy in ST-Segment Elevation Myocardial Infarction Patients — Patient Outcome after Primary Percutaneous Coronary Intervention; TAILOR-PCI, Tailored Antiplatelet Initiation to Lesson Outcomes Due to Decreased Clopidogrel Response After Percutaneous Coronary Intervention. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download