Abstract
The risk of stroke recurrence is highest in the acute phase after transient ischemic attack (TIA) or ischemic stroke. Therefore, patients with TIA or ischemic stroke should be treated with antiplatelet medication for stroke prevention. The short-term use of dual antiplatelet therapy between 21 and 90 days may be considered in those with acute minor stroke or TIA and high-risk of recurrence. However, the long-term use of dual antiplatelet therapy is not recommended due to the risk of bleeding. The current stroke guideline does not specify the administration of an antiplatelet for the secondary prevention of ischemic stroke. However, as clinical studies progress, antiplatelet therapy may become a personalized treatment in the future.
Stroke is a leading cause of mortality and permanent disability worldwide.1) Acute cerebral ischemia such as cerebral infarction and transient ischemic attack (TIA), is often followed by recurrent ischemic events, where recurrent stroke or TIA occurs in 12% of cases at 1 year and 16.8% at 5 years after minor stroke or TIA.2)3) The secondary prevention of recurrent stroke includes the control of risk factors, such as hypertension, diabetes mellitus, and dyslipidemia; lifestyle modification, such as smoking cessation, weight reduction, and regular physical activity; antiplatelet therapy for non-cardioembolic ischemic stroke; and anticoagulation therapy for cardioembolic ischemic stroke.4) This review will focus on the strategy of antiplatelet therapy administration for the secondary prevention of noncardioembolic ischemic stroke or TIA in various situations and time points.
A high-risk of recurrent stroke after TIA or minor stroke exists in the early period, with most strokes occurring within the first 2 days5-8); therefore, more effective preventive treatment modalities are needed at this time. The secondary preventive effect of antiplatelet therapy after ischemic stroke has been well-established. In the past, secondary stroke prevention guidelines recommended the use of single antiplatelet (aspirin, clopidogrel) for initial treatment. Although, the combination of aspirin and clopidogrel is more effective than the use of aspirin alone in reducing the risk of ischemic events in patients with acute coronary syndrome,9) the addition of aspirin to clopidogrel is not recommended for secondary prevention after ischemic stroke or TIA due to the increased risk of bleeding. The MATCH trial randomized patients with a recent TIA or ischemic stroke to clopidogrel plus aspirin versus clopidogrel plus placebo groups. The antiplatelet combination did not reduce major vascular effects, but increased the risk of life threatening events or major bleeding.10) However, this study did not reflect the prevention of recurrent stroke in the early period after the initial event. The mean time from the qualifying event to randomization was 27 days, and subgroup analysis showed a favorable trend of dual antiplatelet agent in the group with early randomization (within 7 days).
The recent stroke secondary prevention guideline recommends that early initiation of short-term (21–90 days) dual antiplatelet therapy with aspirin and clopidogrel (or ticagrelor), ideally within 12–24 hours, and at least within 7 days after the onset of minor stroke (National Institutes of Health Stroke Scale [NIHSS] ≤ 3, NIHSS ≤ 5 for ticagrelor) or high-risk TIA (ABCD2 score ≥ 4, ABCD2 score ≥ 6 for ticagrelor). This guideline is based on three major randomized controlled trials11-13) and meta-analyses14-18) (Table 1). The use of dual antiplatelet therapy with aspirin and clopidogrel beyond 90 days after stroke is not recommended due to the increased risk of intracranial hemorrhage and major bleeding10) (Figure 1).
Single antiplatelet therapy is recommended for acute stroke patients who do not meet the dual antiplatelet clinical criteria (minor stroke or high-risk TIA, intracranial or extracranial arterial stenosis). Several antiplatelet agents, including aspirin (50–325 mg daily) and clopidogrel (75 mg daily), or the combination of aspirin 25 mg and extended-release dipyridamole 200 mg twice daily, are recommended for the secondary prevention of ischemic stroke.19-21)
Intracranial atherosclerosis (ICAS) is a common cause of stroke worldwide,22)23) but is more prevalent in Asian and Black ethnicities than in Caucasians. The annual stroke recurrence rate in patients with ICAS is approximately 4–19%, and it remains as high as 15% in the first year despite optimal medical treatment in several clinical trials comparing the preventive effect of endovascular and medical treatment.24-27)
Several randomized controlled trials have compared percutaneous transluminal angioplasty and stenting (PTAS) with medical therapy for stroke prevention in patients with recent stroke or TIA attributable to severe arterial stenosis. These trials showed a higher rate of cerebrovascular events or death in the PTAS group than in the medical therapy group25)26)28)29) (Table 2).
The stroke prevention guideline recommends the use of dual antiplatelet medication (addition of clopidogrel 75 mg per day to aspirin for up to 90 days) in patients with recent (within 30 days) stroke or TIA attributable to severe (70–99%) ICAS. Further, the guideline recommends the addition of ticagrelor 90 mg to aspirin twice a day for up to 30 days in patients with recent (within 24 hours) minor stroke or high-risk TIA and concomitant ipsilateral >30% stenosis of a major intracranial artery. Although the guideline does not recommend long-term dual antiplatelet therapy beyond 3 months, a previous long-term cohort study reported a higher risk of recurrent stroke in patients with severe (50–99%) ICAS than in those without.30) Additional studies are needed to determine the effect of long-term dual antiplatelet therapy and to establish the specific high-risk subgroup of recurrent stroke in patients with severe symptomatic ICAS.
The medical arm of the SAMMPRIS trial was treated with solely 325 mg aspirin once daily after the first 90 days of dual antiplatelet therapy for the secondary prevention of stroke in severe ICAS, which showed a favorable result compared to interventional arm. The long-term preventive effect of other antiplatelet agents (clopidogrel, cilostazol, ticagrelor, aspirin plus dipyridamole) is not clearly defined and hence should be proven by future studies.
Extracranial carotid artery stenosis is a major risk factor for ischemic stroke. Previous randomized clinical trials have compared carotid endarterectomy (CEA) with optimal medical therapy in patients with recent stroke or TIA.31-34) The stroke guideline recommends that CEA be used to reduce the risk of future stroke in patients with TIA or non-disabling ischemic stroke and ipsilateral severe (50–99%) carotid artery stenosis. Moreover, carotid artery stenting is considered an option for patients at high-risk of CEA complications.
The intensive medical therapies available for the prevention of stroke recurrence in patients with carotid artery stenosis include antiplatelet therapy, lipid-lowering therapy, and treatment of hypertension. The antiplatelet regimens of ongoing clinical trials in patients with carotid artery stenosis include aspirin 325 mg daily in the CREST-2 trial, and combined aspirin and dipyridamole or clopidogrel in the ECST-2 trial.35)36)
Dual antiplatelet therapy is not routinely recommended for the secondary prevention of ischemic stroke with symptomatic carotid artery stenosis, unless the symptoms are minor and within the acute period after symptom onset (described above). The CARESS trial demonstrated that combination therapy with clopidogrel and aspirin was more effective than using aspirin alone in reducing asymptomatic embolization in patients with recent (within 3 months) symptomatic carotid stenosis.37) Although this study used a surrogate marker of microembolic signal instead of clinical outcomes and short duration (7 days) of dual antiplatelet administration, it demonstrated the possible role of dual antiplatelet agent in symptomatic carotid artery stenosis.
Small subcortical strokes, also known as lacunar infarction, comprise approximately onefourth of all cerebral infarctions. The Secondary Prevention of Small Subcortical Strokes (SPS3) study was a clinical trial that investigated the prevention of stroke recurrence by comparing aspirin plus placebo versus dual antiplatelet therapy with aspirin and clopidogrel.38) The addition of clopidogrel to aspirin did not significantly reduce the risk of recurrent stroke but increased the risk of bleeding and death significantly. In the SPS3 trial, the median time from the date of the qualifying stroke to randomization was 62 days; therefore, the result does not reflect the effect of dual antiplatelet medication in acute stage stroke prevention. Lacunar infarction that is detected within 24 hours of symptom onset and minor symptom (NIHSS ≤3) should be treated with dual antiplatelet as recommended by the guideline. The SPS3 trial subgroup analysis showed that in Caucasians receiving aspirin and clopidogrel, patients with cytochrome P450 2C19 (CYP2C19) intermediate or poor metabolizer status had higher probability of recurrent stroke than those with extensive or ultrarapid metabolizer status.39) However, this study is limited by the small proportion of Caucasian participants that showed significant results; thus, the results should be interpreted with caution, and a larger clinical trial is necessary to validate the conclusions.
The American Heart Association stroke guideline recommends antiplatelet therapy in preference to oral anticoagulation to reduce the risk of recurrent ischemic stroke in patients with non-cardioembolic ischemic stroke or TIA.15) Aspirin 50–325 mg daily, clopidogrel 75 mg, or the combination of aspirin 25 mg and extended-release dipyridamole 200 mg twice daily is recommended for the secondary prevention of ischemic stroke, unless there are specific contraindications, such as minor ischemic stroke, high-risk TIA, or stroke due to large vessel occlusive disease.
The ESPRIT and ESPS2 trials showed that the use of aspirin-dipyridamole is more effective than aspirin alone for the prevention of vascular events.20)21) Although the CAPRIE trial was not focused strictly on the secondary prevention of ischemic stroke, it showed fewer composite vascular events among patients treated with clopidogrel than those treated with aspirin; however, there was no benefit in stroke reduction among the stroke subgroup.40) The PRoFESS trial found no significant difference between the use of aspirin-dipyridamole and clopidogrel in terms of secondary stroke prevention after non-cardioembolic stroke.19)
Clopidogrel is widely used as a single or dual combination antiplatelet for secondary prevention in patients with ischemic stroke. The CYP2C19 genotype differentially affects the liver metabolism of clopidogrel, which may influence the preventive effect. Studies on whether the stroke preventive effect differs by CYP2C19 genotype in ischemic stroke patients have shown controversial results. Previous studies demonstrated that reduced or loss of CYP2C19 function was associated with poorer outcome and increased risk of recurrent stroke in patients with ischemic stroke.41)42) In contrast, no significant association between CYP2C19 genotype and ischemic events was found in patients with ischemic stroke.43) In the SPS3 study, there were no significant differences in the occurrence of recurrent stroke by CYP2C19 metabolizer status in the whole study group.39) Moreover, a meta-analysis showed that carriers of CYP2C19 loss-of-function alleles (*2, *3, and *8) were at a 1.92-fold greater risk of stroke than non-carriers.44)
Antiplatelet agents are used for the secondary prevention of cerebral infarction; however, they do not completely prevent the recurrence of cerebral infarction and may have the disadvantage of bleeding as a side effect. Dual antiplatelet therapy is recommended only for patients with acute mild cerebral infarction and cerebral infarction caused by severe arterial stenosis. However, the long-term effect of dual antiplatelet therapy and its use in other situations have not been completely established.
Further studies are needed on the long-term administration of dual antiplatelet agents in patients with cerebral infarction and cerebral artery stenosis or moderate to severe ischemic damage. The clinical implications of antiplatelet resistance, especially clopidogrel, in ischemic stroke prevention, is another important issue that requires further investigation.
REFERENCES
1. GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019; 18:439–58.
2. Amarenco P, Lavallée PC, Labreuche J, Albers GW, Bornstein NM, Canhão P, Caplan LR, Donnan GA, Ferro JM, Hennerici MG, Molina C, Rothwell PM, Sissani L, Školoudík D, Steg PG, Touboul PJ, Uchiyama S, Vicaut É, Wong LKS; TIAregistry.org Investigators. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016; 374:1533–42.

3. Amarenco P, Lavallée PC, Monteiro Tavares L, Labreuche J, Albers GW, Abboud H, Anticoli S, Audebert H, Bornstein NM, Caplan LR, Correia M, Donnan GA, Ferro JM, Gongora-Rivera F, Heide W, Hennerici MG, Kelly PJ, Král M, Lin HF, Molina C, Park JM, Purroy F, Rothwell PM, Segura T, Školoudík D, Steg PG, Touboul PJ, Uchiyama S, Vicaut É, Wang Y, Wong LKS; TIAregistry.org Investigators. Five-year risk of stroke after TIA or minor ischemic stroke. N Engl J Med. 2018; 378:2182–90.

4. Diener HC, Hankey GJ. Primary and secondary prevention of ischemic stroke and cerebral hemorrhage: JACC focus seminar. J Am Coll Cardiol. 2020; 75:1804–18.
5. Johnston SC, Rothwell PM, Nguyen-Huynh MN, Giles MF, Elkins JS, Bernstein AL, Sidney S. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007; 369:283–92.

6. Coull AJ, Lovett JK, Rothwell PM; Oxford Vascular Study. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ. 2004; 328:326.

7. Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in populationbased incidence studies. Neurology. 2004; 62:569–73.

8. Kleindorfer D, Panagos P, Pancioli A, Khoury J, Kissela B, Woo D, Schneider A, Alwell K, Jauch E, Miller R, Moomaw C, Shukla R, Broderick JP. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke. 2005; 36:720–3.

9. Bowry AD, Brookhart MA, Choudhry NK. Meta-analysis of the efficacy and safety of clopidogrel plus aspirin as compared to antiplatelet monotherapy for the prevention of vascular events. Am J Cardiol. 2008; 101:960–6.

10. Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, Leys D, Matias-Guiu J, Rupprecht HJ; MATCH investigators. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004; 364:331–7.

11. Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, Kim AS, Lindblad AS, Palesch YY; Clinical Research Collaboration, Neurological Emergencies Treatment Trials Network, and the POINT Investigators. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018; 379:215–25.

12. Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, Wang C, Li H, Meng X, Cui L, Jia J, Dong Q, Xu A, Zeng J, Li Y, Wang Z, Xia H, Johnston SC; CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013; 369:11–9.

13. Johnston SC, Amarenco P, Denison H, Evans SR, Himmelmann A, James S, Knutsson M, Ladenvall P, Molina CA, Wang Y; THALES Investigators. Ticagrelor and aspirin or aspirin alone in acute ischemic stroke or TIA. N Engl J Med. 2020; 383:207–17.

14. Brown DL, Levine DA, Albright K, Kapral MK, Leung LY, Reeves MJ, Sico J, Strong B, Whiteley WN; American Heart Association Stroke Council. Benefits and risks of dual versus single antiplatelet therapy for secondary stroke prevention: a systematic review for the 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack. Stroke. 2021; 52:e468–79.

15. Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, Kamel H, Kernan WN, Kittner SJ, Leira EC, Lennon O, Meschia JF, Nguyen TN, Pollak PM, Santangeli P, Sharrief AZ, Smith SC Jr, Turan TN, Williams LS. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021; 52:e364–467.

16. Hao Q, Tampi M, O'Donnell M, Foroutan F, Siemieniuk RA, Guyatt G. Clopidogrel plus aspirin versus aspirin alone for acute minor ischaemic stroke or high risk transient ischaemic attack: systematic review and meta-analysis. BMJ. 2018; 363:k5108.

17. Ding L, Peng B. Efficacy and safety of dual antiplatelet therapy in the elderly for stroke prevention: a systematic review and meta-analysis. Eur J Neurol. 2018; 25:1276–84.

18. Patti G, Sticchi A, Bisignani A, Pelliccia F, Pasceri V, Speciale G, Penco M. Meta-regression to identify patients deriving the greatest benefit from dual antiplatelet therapy after stroke or transient ischemic attack without thrombolytic or thrombectomy treatment. Am J Cardiol. 2019; 124:627–35.

19. Sacco RL, Diener HC, Yusuf S, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, Bornstein N, Chan BP, Chen ST, Cunha L, Dahlöf B, De Keyser J, Donnan GA, Estol C, Gorelick P, Gu V, Hermansson K, Hilbrich L, Kaste M, Lu C, Machnig T, Pais P, Roberts R, Skvortsova V, Teal P, Toni D, Vandermaelen C, Voigt T, Weber M, Yoon BW; PRoFESS Study Group. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008; 359:1238–51.

20. ESPRIT Study Group, Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006; 367:1665–73.

21. Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996; 143:1–13.

22. Yaghi S, Prabhakaran S, Khatri P, Liebeskind DS. Intracranial atherosclerotic disease. Stroke. 2019; 50:1286–93.

23. Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. 2008; 39:2396–9.
24. Kim JS, Bang OY. Medical treatment of intracranial atherosclerosis: an update. J Stroke. 2017; 19:261–70.

25. Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, Janis LS, Lutsep HL, Barnwell SL, Waters MF, Hoh BL, Hourihane JM, Levy EI, Alexandrov AV, Harrigan MR, Chiu D, Klucznik RP, Clark JM, McDougall CG, Johnson MD, Pride GL Jr, Torbey MT, Zaidat OO, Rumboldt Z, Clof HJ; SAMMPRIS Trial Investigators. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011; 365:993–1003.

26. Zaidat OO, Fitzsimmons BF, Woodward BK, Wang Z, Killer-Oberpfalzer M, Wakhloo A, Gupta R, Kirshner H, Megerian JT, Lesko J, Pitzer P, Ramos J, Castonguay AC, Barnwell S, Smith WS, Gress DR; VISSIT Trial Investigators. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA. 2015; 313:1240–8.

27. Sangha RS, Naidech AM, Corado C, Ansari SA, Prabhakaran S. Challenges in the medical management of symptomatic intracranial stenosis in an urban setting. Stroke. 2017; 48:2158–63.

28. Compter A, van der Worp HB, Schonewille WJ, Vos JA, Boiten J, Nederkoorn PJ, Uyttenboogaart M, Lo RT, Algra A, Kappelle LJ; VAST investigators. Stenting versus medical treatment in patients with symptomatic vertebral artery stenosis: a randomised open-label phase 2 trial. Lancet Neurol. 2015; 14:606–14.

29. Gao P, Zhao Z, Wang D, Wu J, Cai Y, Li T, Wu W, Shi H, He W, Zhu F, Jiao L, Ling F. China Angioplasty and Stenting for Symptomatic Intracranial Severe Stenosis (CASSISS): a new, prospective, multicenter, randomized controlled trial in China. Interv Neuroradiol. 2015; 21:196–204.

30. Hurford R, Wolters FJ, Li L, Lau KK, Küker W, Rothwell PM; Oxford Vascular Study Phenotyped Cohort. Prevalence, predictors, and prognosis of symptomatic intracranial stenosis in patients with transient ischaemic attack or minor stroke: a population-based cohort study. Lancet Neurol. 2020; 19:413–21.

31. Rothwell PM, Eliasziw M, Gutnikov SA, Fox AJ, Taylor DW, Mayberg MR, Warlow CP, Barnett HJ; Carotid Endarterectomy Trialists' Collaboration. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 2003; 361:107–16.

32. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet. 1998; 351:1379–87.
33. Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, Rankin RN, Clagett GP, Hachinski VC, Sackett DL, Thorpe KE, Meldrum HE, Spence JD. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998; 339:1415–25.

34. Meschia JF, Klaas JP, Brown RD Jr, Brott TG. Evaluation and management of atherosclerotic carotid stenosis. Mayo Clin Proc. 2017; 92:1144–57.

35. Howard VJ, Meschia JF, Lal BK, Turan TN, Roubin GS, Brown RD Jr, Voeks JH, Barrett KM, Demaerschalk BM, Huston J 3rd, Lazar RM, Moore WS, Wadley VG, Chaturvedi S, Moy CS, Chimowitz M, Howard G, Brott TG; CREST-2 study investigators. Carotid revascularization and medical management for asymptomatic carotid stenosis: protocol of the CREST-2 clinical trials. Int J Stroke. 2017; 12:770–8.

36. Stroke Research Group; UCL Institute of Neurology. ECST-2 protocol summary: version 3.10 [Internet]. London: Stroke Research Group, UCL Institute of Neurology;2015. [cited 2021 Aug 31]. Available from: http://s489637516.websitehome.co.uk/ECST2/protocolsummary.
37. Markus HS, Droste DW, Kaps M, Larrue V, Lees KR, Siebler M, Ringelstein EB. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using doppler embolic signal detection: the Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial. Circulation. 2005; 111:2233–40.

38. SPS3 Investigators, Benavente OR, McClure LA, Szychowski JM, Coffey CS, Pearce LA. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012; 367:817–25.

39. McDonough CW, McClure LA, Mitchell BD, Gong Y, Horenstein RB, Lewis JP, Field TS, Talbert RL, Benavente OR, Johnson JA, Shuldiner AR. CYP2C19 metabolizer status and clopidogrel efficacy in the Secondary Prevention of Small Subcortical Strokes (SPS3) study. J Am Heart Assoc. 2015; 4:e001652.

40. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996; 348:1329–39.
41. Lin YJ, Li JW, Zhang MJ, Qian L, Yang WJ, Zhang CL, Shao Y, Zhang Y, Huang YJ, Xu Y. The association between CYP2C19 genotype and of in-stent restenosis among patients with vertebral artery stent treatment. CNS Neurosci Ther. 2014; 20:125–30.

42. Jia DM, Chen ZB, Zhang MJ, Yang WJ, Jin JL, Xia YQ, Zhang CL, Shao Y, Chen C, Xu Y. CYP2C19 polymorphisms and antiplatelet effects of clopidogrel in acute ischemic stroke in China. Stroke. 2013; 44:1717–9.

43. Qiu LN, Sun Y, Wang L, Han RF, Xia XS, Liu J, Li X. Influence of CYP2C19 polymorphisms on platelet reactivity and clinical outcomes in ischemic stroke patients treated with clopidogrel. Eur J Pharmacol. 2015; 747:29–35.

44. Pan Y, Chen W, Xu Y, Yi X, Han Y, Yang Q, Li X, Huang L, Johnston SC, Zhao X, Liu L, Zhang Q, Wang G, Wang Y, Wang Y. Genetic polymorphisms and clopidogrel efficacy for acute ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. Circulation. 2017; 135:21–33.

45. Wang Y, Johnston C, Bath PM, Meng X, Jing J, Xie X, Wang A, Pan Y, Xu A, Dong Q, Wang Y, Zhao X, Li Z, Li H; CHANCE-2 Investigators. Clopidogrel with aspirin in High-risk patients with Acute Non-disabling Cerebrovascular Events II (CHANCE-2): rationale and design of a multicentre randomised trial. Stroke Vasc Neurol. 2021; 6:280–5.

Figure 1.
Antiplatelet therapy for non-cardioembolic stroke and transient ischemic attack.
*Dual antiplatelet is ideally initiated within 24 hours of symptom onset but can be given within 7 days of stroke onset; †Intracranial artery stenosis is defined as stenosis >50%; ‡High-risk TIA is defined as an ABCD2 score ≥4; §The benefit of dual antiplatelet therapy was primarily observed during the first 21 days after symptom onset in major clinical trials; llDual antiplatelet means the combination of aspirin and clopidogrel (modified from the Figure of Stroke 2021;52:e364-46715)).
TIA = transient ischemic attack; NIHSS = National Institutes of Health Stroke Scale.
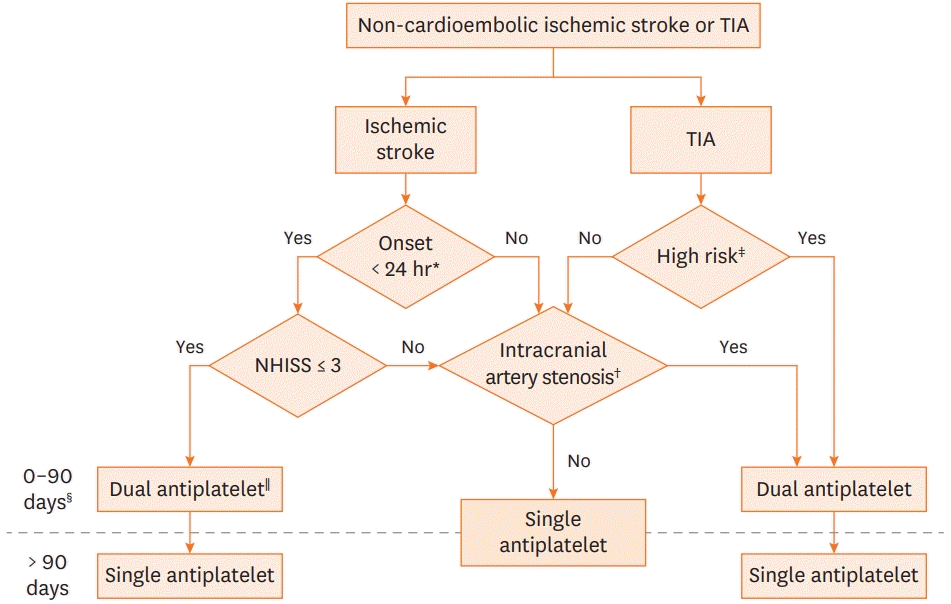
Table 1.
Clinical trials of dual antiplatelet therapy for secondary stroke prevention in acute minor stroke or transient ischemic attack
| Trial | Population | Time window | Antiplatelet intervention | Primary outcome (efficacy & safety) | Key results |
|---|---|---|---|---|---|
| CHANCE12) | 5,170 patients of minor ischemic stroke (NIHSS ≤3) or high-risk TIA (ABCD2 score ≥4) | 24 hours | Clopidogrel (300 mg load then 75 mg/day) plus aspirin 75 mg/day for 21 days then aspirin only vs. aspirin 75 mg/day | Recurrent stroke at 90 days | Reduced recurrent stroke in DAPT (HR, 0.68; p<0.001) |
| Moderate to severe bleeding | No differences in moderate to severe bleeding | ||||
| POINT11) | 4,881 patients of minor ischemic stroke (NIHSS ≤3) or high-risk TIA (ABCD2 score ≥4) | 12 hours | Clopidogrel (600 mg load then 75 mg/day) plus aspirin 50–325 mg/ day vs. aspirin 50–325 mg/day for 90 days | Composite of major ischemic event at 90 days | Reduced major ischemic event in DAPT (HR, 0.75; p=0.02) |
| Major hemorrhage | Increased major hemorrhage in DAPT (HR, 2.32; p=0.02) | ||||
| THALES13) | 11,016 patients of minor ischemic stroke (NIHSS ≤5) or high-risk TIA (ABCD2 score ≥6 or symptomatic arterial stenosis) | 24 hours | Ticagrelor (180 mg load then 90 mg twice daily) plus aspirin (load 300–325 mg then 75–100 mg/day) vs. aspirin (load 300–325 mg then 75–100 mg/day) for 30 days | Composite of stroke or death at 30 days | Reduced stroke or death in DAPT (HR, 0.83; p=0.02) |
| Severe bleeding | Increased severe bleeding in DAPT (HR, 3.99; p=0.001) |
Table 2.
Clinical trials of intracranial stent for secondary stroke prevention
| Trial | Population | Intervention | Outcome | Follow-up duration | Antiplatelet medication for best medical management | Key results |
|---|---|---|---|---|---|---|
| SAMMPRIS25) | 451 patients of TIA or nondisabling stroke with 70–99% stenosis of major intracranial artery | Stenting vs. best medical management | Stroke or death | Mean 11.9 months | Aspirin 325 mg per day for entire follow up and clopidogrel 75 mg per day for 90 days | Stenting resulted in more strokes and death |
| VISSIT26) | 112 patients of hard TIA or stroke with 70–99% stenosis of major intracranial artery | Stenting vs. best medical management | Stroke or hard TIA | 12 months | Aspirin 81–325 mg per day for entire follow up and clopidogrel 75 mg per day for 90 days | Stenting resulted in more strokes and hard TIA |
| VAST28) | 115 patients of TIA or minor stroke with at least 50% stenosis of intra or extracranial vertebral artery | Stenting vs. best medical management | Vascular death, MI, stroke | Median 3 years | Not defined | Stenting did not lower the risk of stroke |
| Discretion of the treating neurologist | ||||||
| CASSISS29) | 380 patients of TIA or stroke with 70–99% stenosis of major intracranial artery | Stenting vs. best medical management | Stroke or death | 36 months | Aspirin 100 mg per day for entire follow up and clopidogrel 75 mg per day for 90 days | On going |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download