INTRODUCTION
DATA SELECTION
AMYLOIDOGENESIS OF TTR PROTEIN
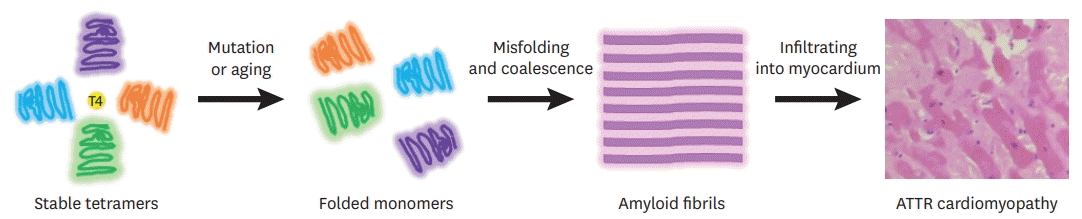 | Figure 1.Schematic diagram of TTR amyloidogenesis. Each monomer consists of 127 amino acids with 1 alpha helix and 8 beta strands. Tetrameric TTR protein has 2 binding sites in a central channel called the T4 pocket; however, the T4 hormone preferentially binds to only 1 of the 2 sites. Stability of TTR tetramers is reduced by mutation or aging processes. As a result, TTR tetramers dissociate into dimeric or monomeric forms. Unstabilized monomers undergo misfolding and aggregation, forming amyloid fibrils.
ATTR = transthyretin amyloidosis; TTR = transthyretin.
|
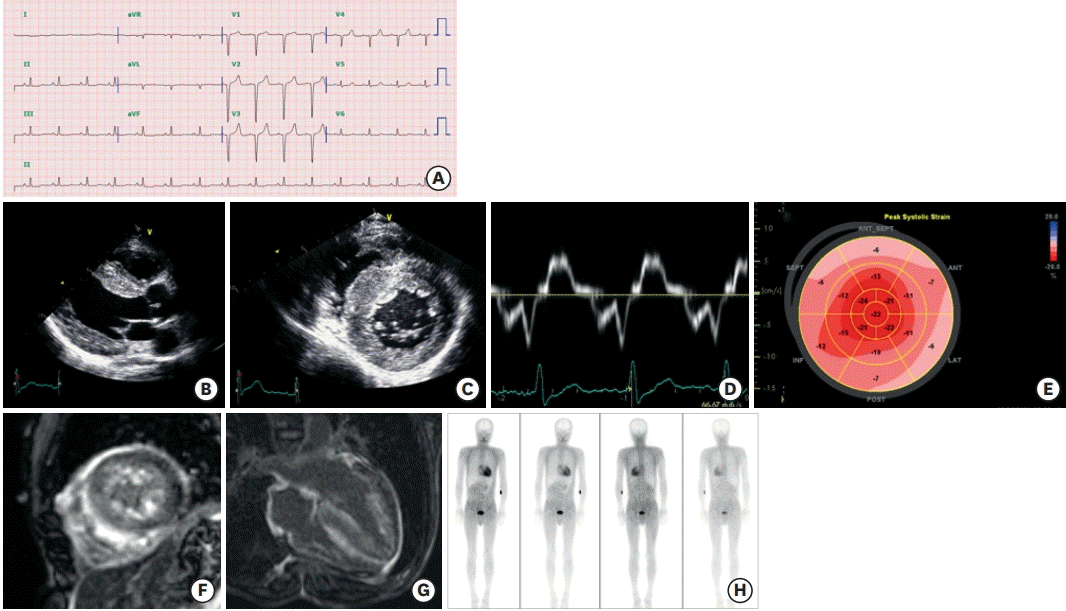 | Figure 2.Clinical features of ATTR cardiomyopathy. A 47-year-old man visited the cardiology clinic complaining of dyspnea on exertion and bilateral pitting edema. The jugular vein was prominent. He had undergone surgery for bilateral carpal tunnel syndrome 3 years ago. He also complained of frequent episodes of diarrhea. The electrocardiography showed a pseudo-infarct pattern (A) and N-terminal prohormone brain natriuretic peptide was 3,600 pg/mL. Transthoracic echocardiography showed a thickened myocardium (15 mm at the septum), a relatively small left ventricle (end-diastolic dimension 45 mm), decreased e′ velocity (4.2 cm/s) with apical sparing of longitudinal strain (B-E). Cardiac magnetic resonance imaging revealed multifocal late gadolinium enhancement with subendocardial ring enhancement (F, G) Tc-99m-3,3-diphosphono-1,2-propanodicarboxylic acid scan showed grade 3 cardiac uptake with increased radioactive uptake in the gastrointestinal tract as well (H). Gene analysis was performed, and Asp58Val mutation was discovered. He was diagnosed with ATTR with involvement of the heart, gastrointestinal tract, and peripheral nerves.
ATTR = transthyretin amyloidosis.
|
Table 1.
| Clinical presentations | |
|---|---|
| Echocardiography | Unexplained increased in wall thickness (>12 mm) with non-dilated LV |
| Thickening of RV free walls, valves, or interatrial septum | |
| Small pericardial effusion | |
| Reduced LV GLS with apical sparing pattern despite preserved ejection fraction | |
| ECG | Pseudo infarct-pattern or low voltage* |
| Labs | Mild increase in troponin levels on repeated occasions |
| Cardiac presentation | Atrioventricular block in presence of increased LV wall thickness |
| Unexplained conduction block needing pacemaker | |
| Elderly HF with preserved ejection fraction refractory to conventional HF therapy | |
| Intolerance to beta blocker, ACEi or ARB | |
| Self-improving hypertension | |
| Low normal blood pressure with previous history of hypertension | |
| Restrictive hemodynamic profile | |
| Extracardiac manifestation | Autonomic signs and symptoms (orthostatic hypotension, alternating constipation/diarrhea, sweating abnormalities) associated with peripheral neuropathy |
| Musculoskeletal symptoms: carpal tunnel syndrome, particularly if bilateral, spinal stenosis |
CLINICAL PHARMACOLOGY
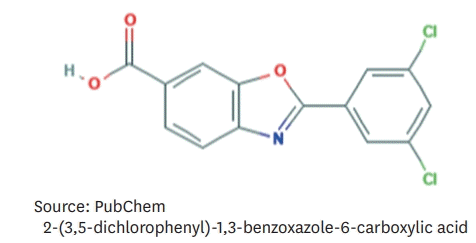 | Figure 3.Molecular structure of tafamidis.14) |
CLINICAL OUTCOMES AND EFFICACY FROM PREVIOUS CLINICAL TRIALS
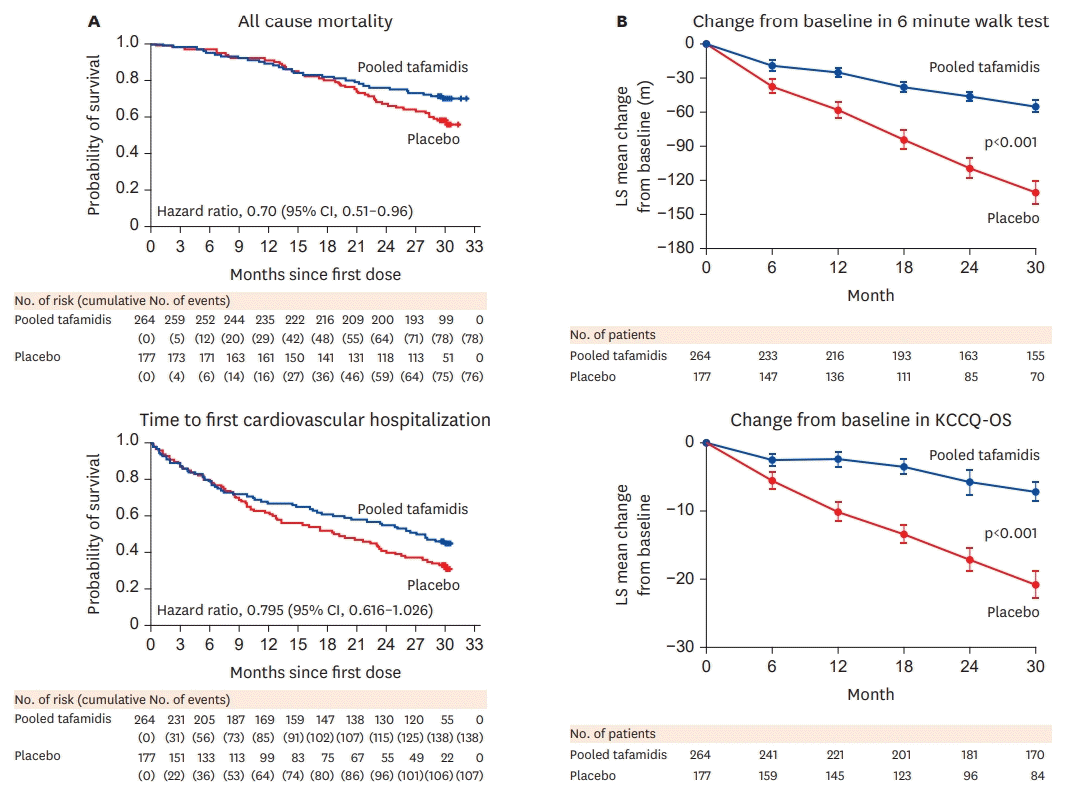 | Figure 4.Clinical outcome in ATTR-ACT trial (modifications of Figures 2 and 4 from 20)) Reduction of all-cause mortality as well as reduction of functional decline was noted in patients treated with tafamidis when compared to those who received placebo.
KCCQ-OS = Kansas City Cardiomyopathy Questionnaire–Overall Summary.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download