Abstract
Background
The CHA2DS2-VASc score is a popular tool for risk prediction of thromboembolism in patients with atrial fibrillation (AF). Each component of the CHA2DS2-VASc scheme is an established risk factor for left ventricular diastolic dysfunction and heart failure (HF). In AF patients, HF is often adversely affecting to clinical outcomes including thromboembolism. We hypothesized that the CHA2DS2-VASc score reflects cardiac reserve and the risk of HF as well as the risk of stroke in patients with AF.
Methods
A total of 103 patients who had the diagnosis of chronic non-valvular AF patients with preserved ejection fraction (EF) were enrolled consecutively. CHA2DS2-VASc score was compared to exercise capacity (peak oxygen uptake, peak VO2), B-type natriuretic peptide (BNP) and echocardiographic diastolic dysfunction index (early mitral to annular velocity, E/E′) ratio.
Results
Exercise capacity was correlated with age (β=−0.568, p<0.001), CHA2DS2-VASc score (β=−0.526, p<0.001), BNP (β=−0.449, p<0.001) and diastolic dysfunction (β=−0.534, p<0.001). Patients with CHA2DS2-VASc score ≥2 had a significantly less exercise capacity than those with CHA2DS2-VASc score <2 (p<0.001). Higher CHA2DS2-VASc score was associated with lower exercise capacity, more diastolic dysfunction and higher BNP (for trend p=0.001).
Conclusions
High CHA2DS2-VASc score is associated with poor exercise capacity in patients with AF. Diastolic dysfunction is thought to be the major mechanism of exercise limitation. CHA2DS2-VASc score might be useful for predicting overall cardiac reserve as well as stroke risk stratification in AF patients.
Stroke and heart failure (HF) are the 2 major problems in patients with atrial fibrillation (AF). Both conditions adversely affect each other. Overt cardiac decompensation or left ventricular (LV) dysfunction is an independent risk factor of stroke, and vice versa.1)2)
The CHA2DS2-VASc score is widely used to predict the risk of thromboembolic stroke and to determine the need for anticoagulation.3)4) Moreover, a high CHA2DS2-VASc score predicts cardiovascular events and mortality.5)6) Although each component of the CHA2DS2-VASc scheme was derived from epidemiologic studies for stroke, it has been confirmed as a risk factor for HF with preserved ejection fraction (EF).7) Whether CHA2DS2-VASc score is correlated with diastolic dysfunction has not been evaluated.
Exercise capacity represents overall cardiovascular fitness. It can be defined by peak oxygen uptake (peak VO2) for a given workload. Peak VO2 is decreased in a variety of cardiovascular illnesses and is a strong prognosticator of cardiovascular and all-cause death.8) As exercise limitation is a critical feature of HF, exercise capacity could also be used to assess overall cardiac functional reserve in patients with AF. We hypothesized that CHA2DS2-VASc score would be correlated with exercise capacity in patients with chronic AF. Cardiac functional parameters including exercise capacity, echocardiographic diastolic function and B-type natriuretic peptide (BNP) were compared to CHA2DS2-VASc score in patients with chronic AF and preserved LVEF.
Consecutive patients with chronic AF were enrolled from August 2013 to October 2014 at a single university hospital. Exclusion criteria were reduced LVEF ≤40%, acute decompensated HF within 30 days, New York Heart Association (NYHA) functional class ≥III, inability or contraindication to treadmill exercise test, evidence of significant coronary heart disease by invasive or non-invasive tests, moderate or severe valvular heart disease, hypertrophic or restrictive cardiomyopathy, moderate or severe obstructive or restrictive lung disease and active malignancy. The primary end point was the relationship between exercise capacity (peak VO2) and CHA2DS2-VASc score. The secondary end point was the relationship between CHA2DS2-VASc score and BNP or echocardiographic diastolic function index. Diagnosis of persistent AF was based on electrocardiogram (ECG) or Holter monitoring at least two times separated by at least 30 days. Definition of components of CHA2DS2-VASc schema followed current practice guidelines.3)4) In brief, congestive HF was defined as history of acute decompensation or pulmonary edema. Hypertension and diabetes mellitus were defined as the need for medication. Vascular disease was defined as the presence of aortic, coronary or carotid arterial disease with significant plaque. Estimated glomerular filtration rate (GFR) was calculated by the Modification of Diet in Renal Disease formula.9) BNP and echocardiography were measured just before cardiopulmonary exercise test. Participants were divided into two groups according to CHA2DS2-VASc score and need for anticoagulation by the current practice guideline.3) Group 1 patients were those with a CHA2DS2-VASc score of 0-1 point and no need for anticoagulation therapy. Group 2 patients had a score ≥2 are required anticoagulation therapy.
Standard echocardiography was performed according to the practice guidelines.10)11) Left atrial volume was measured by the area-length method. LV EF was measured by modified Simpson's method. LV mass was calculated from the M-mode based LV wall thickness and dimension. Early mitral flow velocity (E), mitral annular velocity (E′), and E/E′ ratio were measured according to the standard method. Average of 5 consecutive beats was obtained for all echocardiographic measurements.
A symptom-limited, supervised cardiopulmonary exercise test using a treadmill was performed according to the practice guideline.12) Modified Bruce or Naughton protocol was selected by the supervising physicians' discretion with the intention of achieving peak exercise in about 10 minutes. Beta blockers or non-dihydropyridine calcium channel blockers were stopped at least 72 hours before exercise test. Heart rate and 12-lead ECG were monitored continuously. Baseline systolic and diastolic blood pressure (BP) and heart rate were measured before exercise. BP and heart rate was recorded every one minute throughout the test. Expiratory gas measurements were obtained at rest and during progressive exercise. Breath-by-breath oxygen uptake (VO2), carbon dioxide output (VCO2), minute ventilation (VE) and respiratory gas exchange ratio were calculated and averaged at 20-second intervals. Exercise capacity was defined as peak VO2 during cardiopulmonary exercise test. Ventilatory threshold (VT) was obtained by the V-slope method. VE/VCO2 slope was calculated during submaximal exercise by linear regression. Only data from patients who completed exercise up to a respiratory gas exchange ratio above 1.1 were included for analysis.
Continuous data are presented as mean±standard deviation or median (25th–75th interquartile range). Frequency data are presented as number (%). Student's t-test was used for comparison of continuous variable of 2 different groups. Standardized regression coefficient (β) was used to show the linear correlations of variables. General linear regression model was used to evaluate the significant factors for diastolic dysfunction and exercise capacity. Trend among the patients group of increasing CHA2DS2-VASc score was analyzed with one-way analysis of variance with linear contrast test. The p value <0.05 was considered significant. Statistical analysis was performed using SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA).
A total of 103 patients completed the symptom-limited treadmill cardiopulmonary exercise test up to respiratory gas exchange ratio above 1.1. Modified Bruce protocol was used in 92 (89%) patients. Achieved mean peak VO2 was 24±5 mL/min/kg. There was no adverse cardiovascular or orthopedic event during the test. Baseline demographic, laboratory and echocardiographic data are shown in Table 1. Women were older than men (64±7 vs. 59±8 years, p=0.002) and had higher CHA2DS2-VASc score (1.9±1.1 vs. 3.2±1.2, p<0.001). Hypertension was the most frequent component of CHA2DS2-VASc score in both genders. Patients with high CHA2DS2-VASc score ≥2 (Group 2) were significantly older, more symptomatic and less exercise capacity than those with CHA2DS2-VASc score <2 (Group 1).
Exercise capacity indicated by peak VO2 was correlated with age (β=−0.568, p<0.001), CHA2DS2- VASc score (β=−0.526, p<0.001), BNP (β=−0.449, p<0.001), estimated GFR (β=0.284, p=0.003) and echocardiographic diastolic index indicated by E/E′ ratio (β=−0.534, p<0.001).
Diastolic dysfunction measured by resting echocardiographic E/E′ ratio was correlated with age (β=0.323, p=0.001), CHA2DS2-VASc score (β=0.449, p<0.001), estimated GFR (β=−0.275, p=0.004), BNP (β=0.238, p=0.031), and LVEF (β=−0.263, p=0.007).
CHA2DS2-VASc score was compared to cardiac parameters. Firstly, CHA2DS2-VASc score showed significant negative correlation with peak VO2 (for trend p=0.001, Figure 1A), that is, patients with higher score showed less exercise capacity indicated by lower peak VO2. Secondly, BNP concentration increased accordingly as CHA2DS2-VASc score increased (Figure 1B). Lastly, CHA2DS2-VASc score showed significant positive correlation with echocardiographic diastolic function index of E/E′ ratio (for trend p=0.001, Figure 1C). Patients with higher score had higher E/E′ ratio, that is, more elevated LV filling pressure and diastolic impairment.
The main finding of the present study is that patients with higher CHA2DS2-VASc score showed more severe cardiac functional impairment. CHA2DS2-VASc score seems to reflect overall cardiac function, particularly diastolic function, as the study population had preserved LVEF. It might be used as a general vascular risk index in this patient population.
AF is a major risk factor for stroke and systemic embolism. Such risk depends on many demographic and clinical factors, which constitute the CHA2DS2-VASc scoring system. Components of CHA2DS2-VASc score are consistent with risk factors for HF, particularly diastolic dysfunction. Higher CHA2DS2-VASc scores might indicate the increasing impairment of cardiac performance as well as progressively higher risk of thrombus formation. Several mechanisms contribute to LA thrombus formation and increase the risk of thromboembolism in AF. HF and diastolic dysfunction might enhance such pathogenesis. The loss of atrial kick and atrioventricular synchrony impair adequate ventricular filling. Blood stasis due to incomplete washout of LA blood becomes worse with LV dysfunction, and further promotes thrombus formation.
The current practice guideline recommends a decision for anticoagulation therapy according to the CHA2DS2-VASc score. Patients with a score ≥2 are indicated for anticoagulation. Our result is consistent with this recommendation; diastolic dysfunction and exercise limitation were more prominent in Group 2, which might raise the risk of thromboembolism.
As the CHA2DS2-VASc scheme is an empirical collection of vascular risk factors, it is not surprising that this score is correlated with exercise capacity and cardiac impairment. As well as stroke, the CHA2DS2-VASc score predicts cardiovascular hospitalization and mortality.5)6) Although it was originally designed for non-valvular AF, the score predicted the risk stroke in patients with coronary artery disease who do not have AF.7) In the present study, estimated GFR was correlated with CHA2DS2-VASc score suggesting the probability of the CHA2DS2- VASc score as a systemic vascular risk index. In patients with a high CHA2DS2-VASc score, multiple vascular risk factors would cause chronic endothelial damage in systemic vessels including LV myocardium. A spectrum of cardiovascular disease including LV hypertrophy, diastolic dysfunction, LV filling pressure elevation, LA remodeling and AF would proceed.
AF often coexists with HF in the same patient. Even in patients with lone AF, exercise performance is reduced.13) As well as tachycardia, inadequate LV filling due to atrio-ventricular irrelevance would impair the optimal cardiac performance during exercise. In the present study, LV EF was preserved in the participants and echocardiographic diastolic index of E/E′ ratio showed significant negative correlation with peak VO2. Impaired LV filling looks like an important mechanism of exercise limitation in patients with AF. Our results suggest that patients with poor exercise capacity would have more vascular risk factors and a higher risk of stroke. Poor functional capacity might be useful to predict the future stroke risk scheme.
There are severe limitations in this study. To obtain the reliable indicator of exercise capacity, a great deal of data from patients who could not complete the significant strength exercise were excluded. Therefore, the results do not cover the range of AF patients, particularly those with poor functional capacity and poor LV systolic function. The small number of patients is another important limitation.
In conclusion, high CHA2DS2-VASc score is associated with poor cardiac performance in patients with chronic AF. Diastolic dysfunction appears to the major cause of exercise limitation. CHA2DS2-VASc score might represent the overall cardiac performance as well as stroke risk prediction in patients with AF.
REFERENCES
1. McMurray JJ, Ezekowitz JA, Lewis BS, Gersh BJ, van Diepen S, Amerena J, Bartunek J, Commerford P, Oh BH, Harjola VP, Al-Khatib SM, Hanna M, Alexander JH, Lopes RD, Wojdyla DM, Wallentin L, Granger CB; ARISTOTLE Committees and Investigators. Left ventricular systolic dysfunction, heart failure, and the risk of stroke and systemic embolism in patients with atrial fibrillation: insights from the ARISTOTLE trial. Circ Heart Fail. 2013; 6:451–60.
2. Agarwal M, Apostolakis S, Lane DA, Lip GY. The impact of heart failure and left ventricular dysfunction in predicting stroke, thromboembolism, and mortality in atrial fibrillation patients: a systematic review. Clin Ther. 2014; 36:1135–44.

3. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Ž, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Vardas P, Al-Attar N, Alfieri O, Angelini A, Blömstrom-Lundqvist C, Colonna P, De Sutter J, Ernst S, Goette A, Gorenek B, Hatala R, Heidbüchel H, Heldal M, Kristensen SD, Kolh P, Le Heuzey JY, Mavrakis H, Mont L, Filardi PP, Ponikowski P, Prendergast B, Rutten FH, Schotten U, Van Gelder IC, Verheugt FW; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012; 33:2719–47.
4. Reiffel JA. Atrial fibrillation: what have recent trials taught us regarding pharmacologic management of rate and rhythm control? Pacing Clin Electrophysiol. 2011; 34:247–59.

5. Naccarelli GV, Panaccio MP, Cummins G, Tu N. CHADS2 and CHA2DS2-VASc risk factors to predict first cardiovascular hospitalization among atrial fibrillation/atrial flutter patients. Am J Cardiol. 2012; 109:1526–33.
6. Jover E, Roldán V, Gallego P, Hernández-Romero D, Valdés M, Vicente V, Lip GY, Marín F. Predictive value of the CHA2DS2-VASc score in atrial fibrillation patients at high risk for stroke despite oral anticoagulation. Rev Esp Cardiol (Engl Ed). 2012; 65:627–33.

7. Welles CC, Whooley MA, Na B, Ganz P, Schiller NB, Turakhia MP. The CHADS2 score predicts ischemic stroke in the absence of atrial fibrillation among subjects with coronary heart disease: data from the Heart and Soul Study. Am Heart J. 2011; 162:555–61.

9. Poggio ED, Wang X, Greene T, Van Lente F, Hall PM. Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol. 2005; 16:459–66.

10. Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, Douglas PS, Faxon DP, Gillam LD, Kimball TR, Kussmaul WG, Pearlman AS, Philbrick JT, Rakowski H, Thys DM, Antman EM, Smith SC Jr, Alpert JS, Gregoratos G, Anderson JL, Hiratzka LF, Hunt SA, Fuster V, Jacobs AK, Gibbons RJ, Russell RO; American College of CardiologyAmerican Heart Association; American Society of Echocardiography. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation. 2003; 108:1146–62.
11. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ; Chamber Quantification Writing GroupAmerican Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005; 18:1440–63.

12. Guazzi M, Adams V, Conraads V, Halle M, Mezzani A, Vanhees L, Arena R, Fletcher GF, Forman DE, Kitzman DW, Lavie CJ, Myers J. EACPR/AHA Joint Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J. 2012; 33:2917–27.
Figure 1.
Correlation of CHA2DS2-VASc score and cardiac reserve including exercise capacity. CHA2DS2-VASc score showed significant correlation with exercise capacity indicated by peak VO2 (A), diastolic dysfunction (B), and BNP (C).
BNP = B-type natriuretic peptide; E/E′ = early mitral flow velocity/mitral annular velocityVO2 = oxygen uptake.
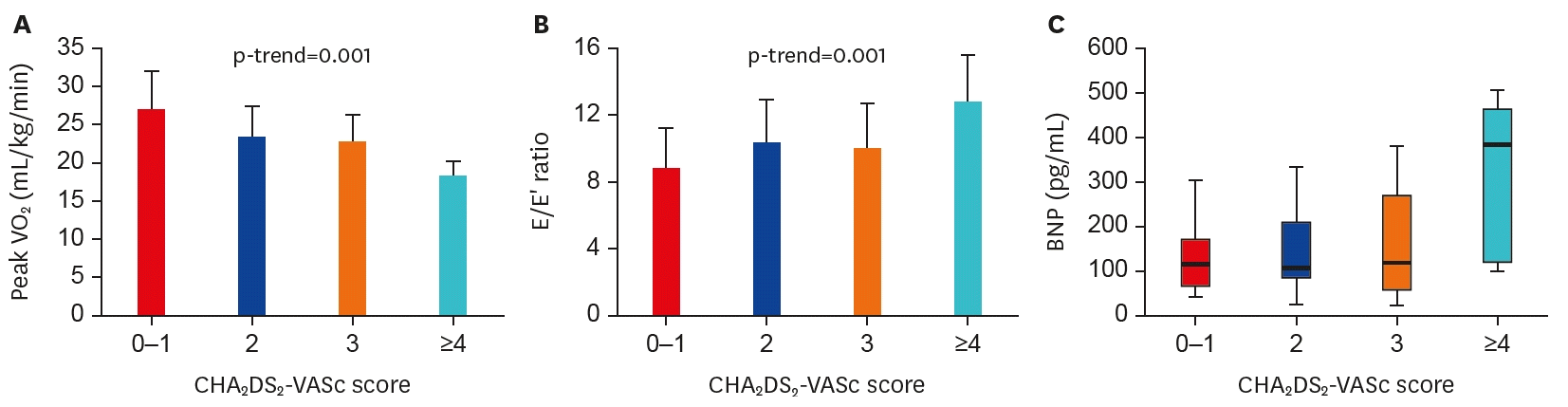
Table 1.
Baseline characteristics
BNP = B-type natriuretic peptide; DBP = diastolic blood pressure; E/E′ = early mitral flow velocity/mitral annular velocity; EF = ejection fraction; GFR = glomerular filtration rate; HR = heart rate; LA = left atrium; LV = left ventricle; NYHA = New York Heart Association; SBP = systolic blood pressure; TIA = transient ischemic attack; VE = minute ventilation; VCO2 = carbon dioxide output; VO2 = oxygen uptake; VT = ventilatory threshold.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download