Abstract
Notes
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2018R1C1B6005448). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
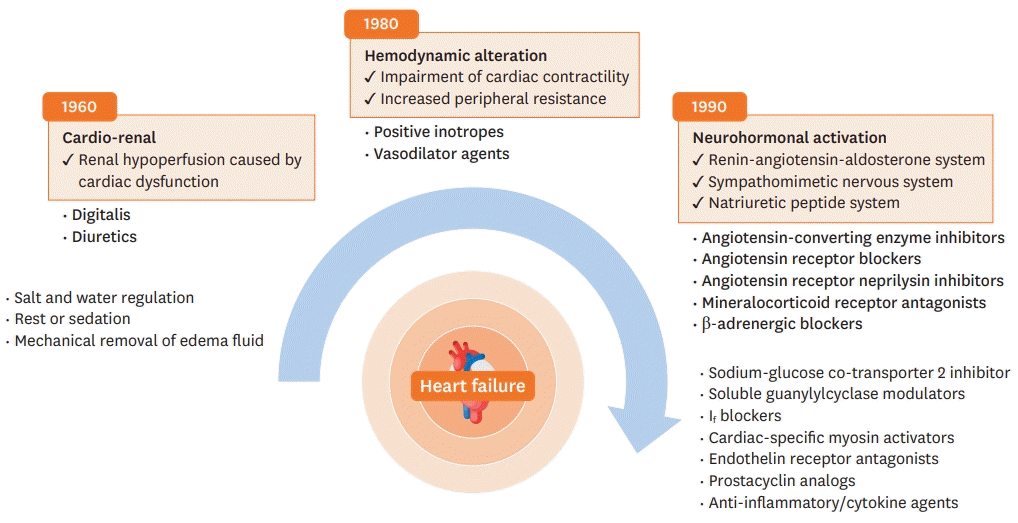
Figure 1.
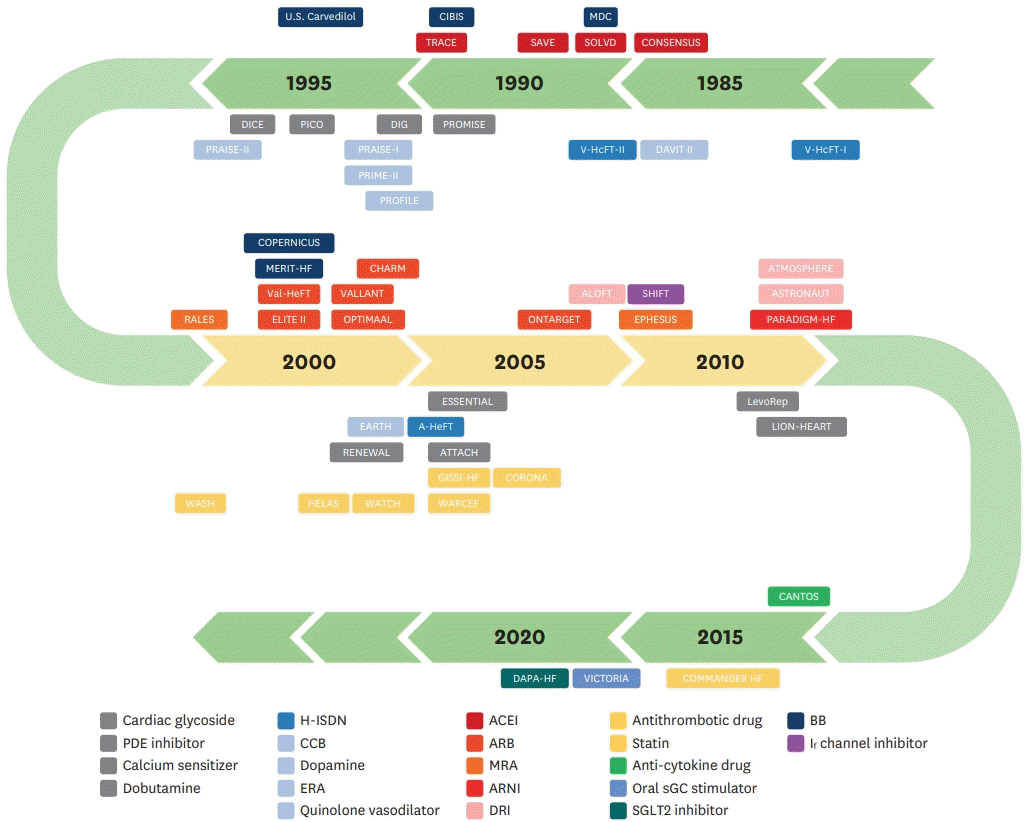
Table 1.
| Study | Year | Participants | Number | Intervention | Comparator | Follow-up | Primary endpoint | Mortality outcome | |
|---|---|---|---|---|---|---|---|---|---|
| ACEIs | |||||||||
| CONSENSUS13) | 1987 | NYHA class IV | 253 | Enalapril | Placebo | Mean 6 momths | All-cause mortality | • Mortality reduced by 40% (p=0.002) at the end of the study | |
| SOLVD14) | 1991 | EF ≤35% | 2,569 | Enalapril | Placebo | Mean 41 months | All-cause mortality | • Mortality reduced by 16% (95% CI, 5% to 26%; p=0.0036) | |
| • Death or hospitalization for CHF reduced by 26% (95% CI, 18% to 34%; p=0.0001) | |||||||||
| SAVE15) | 1992 | Post-MI EF ≤40% without overt HF | 2,231 | Captopril | Placebo | Mean 42 moths | All-cause mortality | • Mortality reduced by 19% (95% CI, 3% to 32%; p=0.0019) | |
| TRACE16) | 1995 | Post-MI EF ≤35% | 1,749 | Trandolapril | Placebo | Mean 36 months | All-cause mortality | • Mortality risk 0.78 (95% CI, 0.67 to 0.91; p=0.001) | |
| ARBs | |||||||||
| ELITE II17) | 2000 | EF ≤40%, NYHA class II–IV | 3,152 | Losartan | Captopril | Median 555 days | All-cause mortality | • No significant differences in all-cause mortality (HR, 1.13; 95.7% CI, 0.95 to 1.35; p=0.16) | |
| Val-HeFT18) | 2001 | NYHA class II–IV ACEIs (93%), BBs (35%) | 5,010 | Valsartan | Placebo | Mean 23 months | Mortality and a combined end point of mortality and morbidity | • Death from any cause (during entire trial) (RR, 1.02; 95% CI, 0.88 to 1.18; p=0.80) | |
| OPTIMAAL19) | 2002 | Post-MI EF <35%, NYHA class I–IV | 5,477 | Losartan | Captopril | Mean 2.7 years | All-cause mortality | • All-cause mortality (HR, 1.13; 95% CI, 0.99 to 1.28; p=0.07) | |
| CHARM-Alternative, Added20), 21) | 2003 | EF ≤40%, NYHA class II–IV | 4,576 | Candesartan | Placebo | Median 40 months | CV death or admission to hospital for management of worsening CHF | • CV death or hospitalization for CHF (HR, 0.82; 95% CI, 0.74 to 0.90; p<0.001) | |
| • All-cause mortality (HR, 0.88; 95% CI, 0.79 to 0.98; p=0.018) | |||||||||
| VALIANT22) | 2003 | Post-MI EF ≤35% | 14,703 | Valsartan | Captopril | Median 24.7 months | All-cause mortality | • All-cause-mortality valsartan vs. captopril (HR, 1.00; 97.5% CI, 0.90 to 1.11; p=0.98) | |
| Valsartan-Captopril | • All-cause-mortality valsartan-captopril vs. captopril (HR, 0.98; 97.5% CI, 0.89 to −1.09; p=0.73) | ||||||||
| ONTARGET23) | 2008 | High-risk patients with CVD or DM, but no HF | 25,620 | Telmisartan | Ramipril | Mean 56 months | Death from CV cause, myocardial infarction, stroke, or hospitalization for heart failure | • Telmisartan vs. ramipril primary outcome (RR, 1.01; 95% CI, 0.94 to 1.09) | |
| Telmisartan-Ramipril | • Telmisartan-ramipril vs. ramipril primary outcome (RR, 0.99; 95% CI, 0.92 to 1.07) | ||||||||
| • Telmisartan-ramipril vs. ramipril total number of discontinuations (RR, 1.20; p<0.0.01) | |||||||||
| BB | |||||||||
| MDC24) | 1993 | HF from idiopathic DCM, EF <40% | 383 | Metoprolol | Placebo | Mean 14 months | All-cause mortality or listing for cardiac transplantation | • Primary endpoint reduced by 34% (95% CI, −6 to 62%; p=0.058) | |
| CIBIS-I25) | 1994 | EF ≤40%, NYHA class III–IV | 641 | Bisoprolol | Placebo | Mean 1.9 years | All-cause mortality | • Mortality (RR, 0.8; 95% CI, 0.56 to 1.15; p=0.22) | |
| U.S. Carvedilol Heart Failure26) | 1996 | CHF EF ≤35% | 1,094 | Carvedilol | Placebo | Median 6.5 months | All-cause mortality | • Mortality reduced by 65% (95% CI, 39 to 80%; p<0.001) | |
| CIBIS-II27) | 1999 | EF ≤35%, NYHA class III–IV | 2,647 | Bisoprolol | Placebo | Mean 1.3 years | All-cause mortality | • All-cause mortality (HR, 0.66; 95% CI, 0.54 to 0.81; p<0.001) | |
| MERIT-HF28) | 1999 | EF ≤40%, NYHA class III–IV | 3,991 | Metoprolol CR/XL | Placebo | Mean 1 years | All-cause mortality | • All-cause mortality (HR, 0.66; 95% CI, 0.53 to 0.81; p<0.001) | |
| COPERNICUS29) | 2001 | EF ≤25%, NYHA class III–IV | 2,289 | Carvedilol | Placebo | Mean 10.4 months | All-cause mortality | • Mortality reduced by 35% (95% CI, 19 to 48%; p=0.0014) | |
| CAPRICORN30) | 2001 | Post-MI EF ≤40% | 1,959 | Carvedilol | Placebo | Mean 1.3 years | All-cause mortality or hospital admission for CV cause | • All-cause mortality (HR, 0.77; 95% CI, 0.60 to 0.98; p=0.03) | |
| MRA | |||||||||
| RALES31) | 1999 | EF ≤35% | 1,663 | Spironolactone | Placebo | Mean 24 months | All-cause mortality | • All-cause mortality (HR, 0.70; 95% CI, 0.60 to 0.82; p<0.001) | |
| EPHESUS32) | 2003 | EF ≤40% | 6,632 | Eplerenone | Placebo | Mean 16 months | All-cause mortality | • Death from any cause (RR, 0.85; 95% CI, 0.75 to 0.96; p=0.008) | |
| Death from CV causes or hospitalization for CV events | • Death from CV causes or hospitalization for CV events (RR, 0.87; 95% CI, 0.79 to 0.95; p=0.002) | ||||||||
| EPMPHASIS-HF33) | 2011 | EF ≤35% NYHA class II | 2,737 | Eplerenone | Placebo | Median 21 months | Death from CV causes or a first hospitalization for HF | • Primary outcome (HR, 0.63; 95% CI, 0.54 to 0.74; p<0.001) | |
| ISDN | |||||||||
| V-HeFT-I34) | 1986 | EF ≤45% | 642 | H-ISDN | Prazosin | Mean 2.3 years | All-cause mortality | • Mortality reduced by 34% (p=0.028) | |
| Placebo | |||||||||
| V-HeFT-II35) | 1991 | EF ≤45% | 804 | H-ISDN | Enalapril | Mean 2.3 years | All-cause mortality | • Mortality reduced by 28% in enalapril (p=0.0016) | |
| A-HeFT36) | 2004 | EF ≤45%, NYHA class III–IV | 1,050 | H-ISDN | Placebo | Mean 10 months | Death from any cause, a first hospitalization for HF, change in QOL | • Death from any cause (HR, 0.57; p=0.01) | |
| African-American | |||||||||
| If channel inhibitor | |||||||||
| SHIFT37) | 2010 | EF ≤35% | 6,558 | Ivabradine | Placebo | Mean 2.5 years | CV death or hospital admission for worsening HF | • Primary outcome (HR, 0.82; 95% CI, 0.75 to 0.90; p<0.0001) | |
| Sinus rhythm with heart rate >70 bpm | • CV death (HR, 0.91; 95% CI, 0.80 to 1.03; p=0.128) | ||||||||
| • Death from HF (HR, 0.74; 95% CI, 0.58 to 0.94; p=0.014) | |||||||||
| • All-cause-death (HR, 0.90; 95% CI, 0.80 to 1.02; p=0.092) | |||||||||
| ARNI | |||||||||
| OVERTURE38) | 2002 | EF ≤30%, NYHA class II–IV | 5,770 | Omapatrilat | Enalapril | Mean 14.5 months | All-cause mortality or hospitalization for HF | • Primary outcome (HR, 0.94; 95% CI, 0.86 to 1.03; p=0.187) | |
| PARADIGM-HF39) | 2014 | EF ≤40%, NYHA class II–IV | 8,442 | Sacubitril–Valsartan | Enalapril | Median 27 months | Death from CV causes or hospitalization for HF | • Primary outcome (HR, 0.80; 95% CI, 0.73 to 0.87; p<0.001) | |
| • All-cause death (HR, 0.84; 95% CI, 0.76 to 0.93; p<0.001) | |||||||||
| SGLT inhibitor | |||||||||
| DAPAHF40) | 2019 | EF ≤40%, NYHA class II–IV | 4,744 | Dapagliflozin | Placebo | Median 18.2 months | Worsening HF or CV death | • Primary outcome (HR, 0.74; 95% CI, 0.65 to 0.85; p<0.001) | |
| • CV death or HF hospitalization (HR, 0.75; 95% CI, 0.65 to 0.85; p<0.001) | |||||||||
| • All-cause death (HR, 0.83; 95% CI, 0.71 to 0.97; p=NA) | |||||||||
| Oral sGC stimulator | |||||||||
| VICTORIA41) | 2020 | EF ≤45%, NYHA class II–IV | 5,050 | Vericiguat | Placebo | Median 10.8 months | Death from CV causes or first hospitalization for HF | • Primary outcome (HR, 0.90; 95% CI, 0.82 to 0.98; p=0.02) | |
| • All-cause death or first hospitalization for HF (HR, 0.90; 95% CI, 0.83 to 0.98; p=0.02) | |||||||||
| • All-cause death (HR, 0.95; 95% CI, 0.84 to 1.07; p=0.38) | |||||||||
ACEI = angiotensin-converting-enzyme inhibitor; ARB = angiotensin II receptor blocker; ARNI = angiotensin receptor-neprilysin inhibitor; BB = beta-blocker; CHF = chronic heart failure; CI = confidence interval; CR/XL = controlled-release/extended-release preparation; CV = cardiovascular; CVD = cardiovascular disease; DCM = dilated cardiomyopathy; DM = diabetes mellitus, EF = ejection fraction; HF = heart failure; HR = hazard ratio; H-ISDN = hydralazine-isosorbide dinitrate; MI = myocardial infarction; NYHA = New York Heart Association; RR = relative risk; QOL = quality of life; sGC = soluble guanylate cyclase; SGLT = sodium-glucose co-transporter 2.
Table 2.
| Target | Study | Year | Participants | Number | Follow-up | Results | |
|---|---|---|---|---|---|---|---|
| Cardiostimulatory drugs | |||||||
| Dopamine | DICE55) | 1999 | NYHA class III–IV, EF ≤30% | 38 | 6 months | Intermittent low-dose dobutamine did not improve the functional status or mortality rate compared with placebo | |
| Amrinone | Massie et al.56) | 1985 | NYHA class III–IV | 99 | 12 weeks | Adverse reactions were significantly more frequent and more severe with amrinone, occurring in 83% of patients | |
| Milrinone | PROMISE57) | 1991 | NYHA class III–IV, EF ≤35% | 1,088 | Median6.1 months | There was a 28% increase in all-cause mortality with milrinone therapy compared with placebo | |
| Enoximone | ESSENTIAL58) | 2009 | NYHA class III–IV, EF ≤30% | 1,854 | Median 16.6 months | Composite of time to all-cause mortality or CV hospitalization did not differ compared with placebo | |
| Vesnarinone | Jay et al.59) | 1998 | NYHA class III–IV, EF ≤30% | 3,833 | Mean 9.5 months | Dose-dependent increase in mortality with vesnarinone compared with placebo | |
| Pimobendan | PICO60) | 1996 | NYHA class II–III, EF ≤45% | 317 | 24 weeks | In both pimobendan groups (2.5 and 5 mg) combined the hazard of death was 1.8 times higher compared with placebo | |
| Levosimendan | LevoRep61) | 2014 | NYHA class III–IV, EF ≤35% | 120 | 24 weeks | Pulsed infusions of levosimendan did not significantly improve functional capacity or quality of life compared with placebo | |
| Levosimendan | LION-HEART62) | 2018 | NYHA class III–IV, EF ≤35% | 69 | 12 weeks | Pulsed infusions of levosimendan significantly reduced NT-proBNP levels and risk of hospitalization | |
| Reduction of all-cause death was not statistically significant compared with placebo | |||||||
| Digoxin | DIG63) | 1997 | EF ≤45% | 3,397 | Mean 37 months | Reduced rate of hospitalization both overall and for worsening HF (RR, 0.72) compared with placebo | |
| Insignificant reduction in overall mortality (RR, 0.99; p=0.80) | |||||||
| Drugs with a vasodilatory effect | |||||||
| Nifedipine | Elkayam et al.64) | 1990 | NYHA class II–III, EF ≤40% | 28 | 8 weeks | Significantly higher incidence of clinical deterioration and worsening of CHF was observed with nifedipine alone or in combination with isosorbide dinitrate compared with placebo | |
| Verapamil | DAVIT II65) | 1990 | Post MI | 1775 (HF: 614) | Mean 16 months | Verapamil did not improve mortality or hospitalization for HF patients compared with placebo | |
| Diltiazem | Goldstein et al.66) | 1991 | Post MI, Baseline EF ≤40% | 623 | Mean 25 months | Diltiazem was associated with enhanced CHF after MI in patients with preceding LV dysfunction compared with placebo | |
| Amlodipine | PRAISE67) | 1996 | NYHA class III–IV, EF ≤30% | 1,153 | Median 13.8 months | No difference between the amlodipine and placebo groups in the occurrence of all-cause death and hospitalization for major CV events | |
| Amlodipine | PRAISE II68) | 2013 | NYHA class III–IV, EF ≤30% | 1,654 | Median 33 months | Amlodipine had no benefits on clinical outcomes for patients with HF, regardless of underlying coronary artery disease compared with placebo | |
| Nonischemic cardiomyopathy | |||||||
| Felodipine | V-HeFT III69) | 1997 | EF ≤45% | 450 | Mean 18 months | Trend for long-term beneficial effects in exercise tolerance and depression for quality of life | |
| Ibopamine | PRIME II70) | 1997 | NYHA class III–IV, EF ≤35% | 1,906 | Mean 347 days | Increased the risk of death among patients (RR 1.26, p=0.017) treated with ibopamine compared with placebo | |
| Darusentan | EARTH71) | 2004 | NYHA class II–IV, EF ≤35% | 642 | 24 weeks | No benefit in cardiac remodeling, clinical symptoms, or outcomes compared with placebo | |
| Bosentan | Packer et al.72) | 2017 | NYHA class III–IV, EF ≤35% | 1,613 | Median 1.5 years | No improvement in clinical outcomes and increased fluid retention within the first 2–4 weeks compared with placebo | |
| Flosequinan | Packer et al.73) | 2017 | NYHA class III–IV, EF ≤35% | 2,354 | Median 10 months | Increased risk of death in patients treated with flosequinan compared with placebo (HR 1.39, p=0.0006) | |
| RAAS modulators | |||||||
| Aliskiren | ALOFT74) | 2008 | NYHA class II–IV | 302 | 3 months | Reduced plasma BNP and urinary aldosterone with aliskiren | |
| Plasma BNP >100 pg/mL | No effect on blood pressure or biochemistry compared with placebo | ||||||
| Aliskiren | ASTRONAUT75) | 2013 | NYHA class II–IV, EF ≤30% | 1,615 | Median 11.3 months | No reduction in CV death or HF rehospitalization compared with placebo | |
| BNP ≥400 pg/mL or NT-proBNP ≥1,600 pg/mL NYHA class II–IV, EF ≤35% | No benefit of aliskiren added to enalapril but increased adverse events | ||||||
| Aliskiren | ATMOSPHERE76) | 2016 | BNP ≥150 pg/mL or NT-proBNP ≥600 pg/mL | 2,336 | Median 36.6 months | Aliskiren did not show noninferiority compared with enalapril | |
| Antithrombotic drugs | |||||||
| Aspirin | WASH77) | 2004 | EF ≤35% | 279 | Mean 27 month | No benefit in clinical outcome compared with placebo | |
| Warfarin | More patients in the aspirin group were hospitalized for worsening HF | ||||||
| Aspirin | HELAS78) | 2006 | EF <35% | 197 | Mean 21.9 months | No clinical benefit of aspirin or warfarin compared with placebo | |
| Warfarin | Ischemic heart disease (aspirin) | ||||||
| Dilated cardiomyopathy (warfarin) | |||||||
| Exclusion: Atrial fibrillation | |||||||
| Aspirin | WATCH79) | 2009 | NYHA class II–IV, EF ≤35% | 1,587 | Mean 1.9 years | No statistical difference between aspirin, clopidogrel, and warfarin on mortality risk | |
| Clopidogrel | Normal sinus rhythm | ||||||
| Warfarin | |||||||
| Aspirin | WARCEF80) | 2012 | EF ≤35% | 2,305 | Mean 3.5 years | No significant reduction on composite end point of ischemic stroke, intracerebral hemorrhage, or death from any cause between aspirin and warfarin | |
| Warfarin | Normal sinus rhythm | ||||||
| Rivaroxaban | COMMANDER HF81) | 2018 | EF ≤40% | 5,022 | Median 21.1 months | No significant reduction in the rate of death, myocardial infarction, or stroke compared with placebo | |
| BNP ≥200 pg/mL or NTproBNP ≥800 pg/mL | |||||||
| Exclusion: Atrial fibrillation | |||||||
| Statins | |||||||
| Rosuvastatin | CORONA82) | 2007 | NYHA class II–IV Age ≥60 years | 5,011 | Median 32.8 months | No reduction in mortality in the rosuvastatin group, but fewer hospitalization compared with placebo (p<0.001) | |
| Rosuvastatin | GISSI-HF83) | 2008 | NYHA class II–IV | 4,631 (EF ≤40%: 4,113) | Median 3.9 years | Rosuvastatin had no benefit on clinical outcomes | |
| including the subgroup with EF ≤40% compared with placebo | |||||||
| Anti-cytokine drugs | |||||||
| Infliximab | ATTACH84) | 2003 | NYHA class III–IV, EF ≤35% | 150 | 28 weeks | No clinical benefit, and higher dose of infliximab (10 mg/kg) increased the combined risk of death from any cause or hospitalization compared with placebo | |
| Etanercept | RENEWAL85) | 2004 | NYHA class II–IV, EF ≤30% | 1,123 (RECOVER) | 24 weeks | Etanercept had no effect on death or hospitalization due to CHF | |
| 925 (RENAISSANCE) | |||||||
| Canakinumab | CANTOS86) | 2019 | Prior MI | 10,061 (HF: 2,173) | Median 3.7 years | Dose-dependent reduction in hospitalization for HF and the composite of hospitalization for HF or Hrrelated mortality compared with placebo | |
| hsCRP ≥2 mg/L | |||||||
BNP = B-type natriuretic peptide; CHF = chronic heart failure; CV = cardiovascular; EF = ejection fraction; HF = heart failure; HR = hazard ratio; hsCRP = highsensitivity C-reactive protein; LV = left ventricular; MI = myocardial infarction; NT-proBNP = N-terminal pro-B-type natriuretic peptide; NYHA = New York Heart Association; RAAS = renin-angiotensin-aldosterone system; RR = relative risk.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download