Abstract
The number of patients with type 2 diabetes (T2D) is increasing worldwide and that in Korea, particularly, has shown an exponential increase with a rise in the older population. The diabetic population is predicted to soar up to 6 million by 2050. The prevalence of diabetes among Korean adults is approximately 15%, while that of prediabetes is 25%, with a total prevalence of 40%. As 40% of the prediabetes cases subsequently progress to T2D, prevention through proactive interventions at the prediabetes stage is essential to reduce the socioeconomic burden due to T2D and the complications of diabetes. With regard to the prevention of T2D, new findings have been published related to the implementation of lifestyle interventions such as exercise and diet as well as drug treatments and surgeries, which have deepened our understanding of the prevention of T2D. Based on published evidence, this review aimed to examine the methods used in the prevention of diabetes.
In most cases, the key pathophysiological features of type 2 diabetes (T2D) include insulin resistance and relatively impaired insulin secretion. Most patients show a natural progression from prediabetes characterized by insulin resistance and consequent glucose intolerance to T2D. An elevated level of insulin is a characteristic of prediabetes; as the level starts to decline, the postprandial blood glucose level rises with an increase in the rate of gluconeogenesis in the liver, which, ultimately, leads to impaired fasting glycemia. The reduced insulin secretion is presumed to be caused by both inherited and acquired factors. Evidently, the prediabetes stage is the target of intervention to prevent diabetes because its natural progression is relatively well characterized while it progresses to diabetes.
The interventions for diabetes prevention include lifestyle modification such as diet and exercise therapies as well as drug therapy. For the primary prevention of diabetes, it is crucial to determine which individuals belong to the high-risk group. However, there is no large-scale study that has clearly identified the age at which diabetes screening test should be performed, the frequency of the screening test, or the cost-effectiveness of the screening test.
The first diabetes screening test should be performed at the age of ≥40 years. In the presence of a risk factor additional to the ones presented in Table 1, the screening test should be carried out at the age of 30 years. If the test result is normal, then the screening test should be performed in 3-year intervals.
The major risk factors for diabetes are obesity and insufficient exercise. Psychological stress and depression are also associated with increased incidence of T2D. The risk factors that cannot be corrected include family history of T2D, history of gestational diabetes, and aging, which are helpful in screening the individuals who are likely to benefit from the Diabetes Prevention Program (DPP).
The incidence of obesity, in particular, has recently shown an exponential increase, in association with the increase in the risk of diabetes. The relationship between obesity and the risk of diabetes is well known. Central obesity shows a particularly close association with insulin resistance, as it increases the risk of T2D. Compared with other adipose tissues, the visceral adipose tissue has a higher metabolic turnover. Free fatty acids flow directly into the liver through the portal vein. The increased rate of lipid degradation in the visceral fat exposes the liver to a high concentration of free fatty acids, which increases the risk of insulin resistance and gluconeogenesis in the liver. Thus, visceral fat reduction and the consequent weight reduction can prevent progression to diabetes in patients with impaired glucose tolerance.
The association between reduced physical activity and obesity is well known. For instance, individuals with an occupation that requires physical labor conventionally show low prevalence of T2D. However, these people tended to move away from the traditional lifestyle and rapidly acquired the urbanized lifestyle, which increased the prevalence of diabetes. For people with a modern lifestyle, reduced physical activity because of spending increased time on watching television or using smartphone is a global trend. Physical exercise directly increases the insulin-mediated glucose uptake in the skeletal muscle, thereby improving insulin resistance. In a study involving Caucasian men, an increase in exercise intensity was shown to reduce the risk of diabetes regardless of age or weight.
The Finnish Diabetes Prevention Study was conducted by 5 research centers in Finland, recruiting 522 obese patients diagnosed with impaired glucose tolerance, to investigate whether a change in lifestyle could prevent the progression to T2D.1) The control group was provided with general instructions regarding diet and exercise, while the treatment group was provided with individualized instructions. Results of the monitoring conducted during the 3.2-year follow-up showed that the incidence of diabetes was reduced by approximately 58% in the treatment group; particularly, patients with a change in lifestyle showed more distinct results. In the treatment group, patients who achieved 4 or more of the 5 suggested goals did not show any incidence of diabetes, while those who had failed to achieve the goals showed a similar incidence as that of the control. The mean weight loss in the treatment group after 2 years was 3.5 kg, indicating the importance of even a small amount of weight loss.
Da Qing conducted a study among patients with impaired glucose tolerance; after a 6-year intervention, the risk of diabetes decreased to 31% in the diet therapy group, 46% in the exercise therapy group, and 42% in the diet and exercise combined therapy group.2) The combination of diet and exercise did not lead to additional advantages. In patients who did not develop diabetes, varying changes in weight were reported, ranging from 0.93 kg to 1.77 kg. Considering such small change in weight, the key contributing factor in this study is thought to be the exercise therapy.
The DPP was conducted by 27 research centers in the United States on 3,234 patients diagnosed with impaired glucose tolerance. This study aimed to investigate whether lifestyle modification or metformin administration could prevent progression to T2D3) by dividing patients into 3 groups: the active lifestyle modification group, the standard lifestyle + metformin administration group, and the standard lifestyle + placebo group. Results of the 2.8-year follow-up monitoring showed that the incidence of diabetes was reduced by 58% in the active lifestyle modification group compared with that in the standard lifestyle + placebo group, wherein 50% of the patients displayed a weight loss of 7% or more with 74% having maintained moderate exercise of at least 150 minutes per week. Notably, the effect of active lifestyle modification was prominent in older adults aged ≥60 years and the incidence of diabetes decreased by 71%.
In the post-hoc analysis of 12 randomized controlled studies and 5,238 patients, the incidence of diabetes was 25.7% in the control group, but it was 14.8% in the lifestyle intervention group with simultaneous diet and exercise therapy. This finding indicated that the lifestyle intervention decreased the incidence of diabetes by 43% (relative risk ratio, 0.57; 95% confidence interval [CI], 0.50, 0.64).4)
In the Diabetes Prevention Program Outcomes Study that followed the DPP, an additional follow-up study of an average of 5.7 years was conducted after terminating the 2.7-year study in the 3 patient groups: control, lifestyle intervention, and metformin administration groups. All 3 groups were provided with a group lifestyle intervention, while metformin treatment was still administered in the metformin administration group. The results showed that the incidence of T2D was similar among the 3 groups: 5.9 (5.1–6.8) of 100 patients per year in the metformin administration group, 4.9 (4.2–5.7) in the lifestyle intervention group, and 5.6 (4.8–6.5) in the control group.5) However, for the 10-year cumulative incidence, the lifestyle intervention group showed a 34% lower incidence of diabetes. Through lifestyle intervention, the incidence of new-onset diabetes could be prevented or delayed for at least 10 years.
In the additional 15-year follow-up study, the diabetes incidence was reduced by 27% in the lifestyle intervention group compared with that in the control group (risk ratio, 0.73; 95% CI, 0.65, 0.83; p<0.001). The intergroup difference showed a decreasing trend over time. For women, the lifestyle intervention was found to have reduced the microvascular complications (intervention: 8.7% [95% CI, 7.4, 10.2] vs. placebo: 11.0% [95% CI, 9.6, 12.6]).6)
In the Finnish Diabetes Prevention Study, an additional 3-year follow-up was conducted after terminating the 4-year intervention monitoring. In the follow-up study, even after terminating the individual lifestyle consultations, the incidence of new-onset diabetes decreased by 43% in the initial lifestyle intervention group: for the new incidence of T2D, the intervention group showed 4.5 out of 100 persons per year, while the control group showed 7.4 out of 100 persons per year; p=0.001.7) In the previous lifestyle intervention group, even after terminating the study, the patients showed a lower level of total fat and saturated fat intakes and higher dietary fiber intake, but the level of activity remained high; hence, the patients' weight remained low.
In the additional 9-year follow-up study to the 4-year intervention monitoring of the Finnish Diabetes Prevention Study, the effect of diabetes prevention was maintained.8) In the initial lifestyle intervention group, the incidence of new-onset diabetes decreased by 32% (relative risk ratio, 0.672; 95% CI, 0.477, 0.947; p=0.023). The patients in the initial lifestyle intervention group were able to maintain a healthy lifestyle with a lower weight. The lifestyle intervention provided to the patients with higher risk of T2D showed a long-term preventive effect.
A similar effect was observed in the study on Asian individuals. Da Qing conducted a 14-year follow-up study after terminating the 6-year intervention. During the follow-up, the lifestyle intervention group showed a lower incidence of diabetes (43%) than the control group (risk ratio, 0.57; 95% CI, 0.41, 0.81). In the lifestyle intervention group, the average time taken until the incidence of diabetes was delayed by 3.6 years on average.9) In the 23-year follow-up study conducted by Da Qing, the incidence of new-onset diabetes decreased by 45% (risk ratio, 0.55; 95% CI, 0.40, 0.76; p=0.001). The risk ratio of the mortality due to a cardiovascular disease was 0.59 (95% CI, 0.36, 0.96; p=0.033).10)
What is most important in the prevention of T2D is to reduce the caloric intake.11) The nutritional counseling should focus on shaping healthy dietary habits rather than on the intake of specific nutrients. The risk of diabetes is influenced by certain dietary intake. Several studies have consistently reported that the risk of incidence of new-onset diabetes was increased by ingestion of refined grains, processed red meat (ham), and beverages containing simple sugars, whereas the risk was decreased by consumption of vegetables, fermented yogurts, nuts, berries, and coffee.11-13) A previous randomized controlled study showed that the individualized medical nutritional therapy effectively reduced the level of glycated hemoglobin.14) Diet therapy education should not only be individualized but also incorporate personal, cultural, and social factors.
Recent studies have reported that the type of fat in the diet is more important than the amount of fat. In the study conducted by Jacobs who conducted a 10-year follow-up monitoring of 166,550 multiracial cohorts (Caucasian, African-American, Hawaiian, Japanese, and Hispanic), 9,200 individuals had new-onset diabetes. The diet quality parameters showed an association with low incidence of diabetes (13–28%).15) A meta-analysis of a prospective cohort study showed that the Mediterranean diet rich in monosaturated fat led to the prevention of T2D by 29% (risk ratio, 0.71; 95% CI, 0.56, 0.90).16) In a prospective, randomized controlled study, a 4-year follow-up monitoring was conducted on 418 individuals divided into 3 groups: the low-fat diet group (the control group), the Mediterranean diet + olive oil (1 L/week) group, and the Mediterranean diet + nuts (30 g) group. Compared with the control group, the risk of new-onset diabetes in the Mediterranean diet groups reduced by approximately 50% even after correction of multivariate variables.17) The risk ratios were 0.49 (0.25–0.97) and 0.48 (0.24–0.96), respectively. In a meta-analysis comprising nine prospective cohort studies and one clinical study on 13,646 individuals, those showing a high level of adherence to the Mediterranean diet had a 23% decrease in the new incidence of T2D (risk ratio, 0.77; 95% CI, 0.66, 0.89).18)
In a meta-analysis comprising 18 cohort studies on 540,184 individuals, the intake of docosahexaenoic acid and eicosapentaenoic acid prevented the incidence of new diabetes (risk ratio, 1.04 for the intake of 250 mg/day; 95% CI, 0.97, 1.10).19) Nonetheless, the intake of omega-3 through consumption of fish increased the risk of diabetes in the western countries; in the eastern countries, the risk was shown to have been decreased. A meta-analysis comprising 20 randomized controlled studies on T2D patients also showed that the supplementary intake of omega-3 had not reduced the level of glycated hemoglobin.20)
Numerous studies consistently showed that fruits had no association with the risk of diabetes.21-23) In a meta-analysis comprising 3 prospective cohort studies, the risk of diabetes was reduced by consumption of blueberries, grapes, and apples.21) Compared with the individuals with an intake of less than 4 servings of such fruits per week, those with an intake of 3 or more servings showed the following relative risk ratio for each fruit: apples, 0.82 (0.73–0.92); grapes, 0.77 (0.64–0.92); and blueberries, 0.82 (0.69–0.98). On the contrary, no preventive effect was reported for consumption of peaches, bananas, oranges, and strawberries.22) In another cohort study (2,332 individuals, 20-year follow-up monitoring), the risk of diabetes was reduced by 35% in the individuals consuming a diet rich in berries (relative risk ratio, 0.65; 95% CI, 0.49, 0.88; p=0.003). However, the cohort studies described had not targeted the prediabetic patients but focused on the general population; therefore, it remains unclear whether the intake of fruits can reduce the risk of diabetes in prediabetic patients. Likewise, there is no evidence to suggest that the intake of fruits increases the risk of diabetes in prediabetic patients. Because fruit intake above a certain level is restricted for diabetic patients, the intake of certain fruits cannot be recommended yet to prediabetic patients.
The supplementary intake of vitamins or minerals is not recommended in patients with T2D or can prevent T2D unless the patient exhibits vitamin or mineral deficiency. Nevertheless, a balanced diet should be recommended to ensure adequate vitamin intake. In a meta-analysis comprising a prospective study, a reverse correlation was found between the plasma level of 25-hydroxy Vit D and the incidence of new-onset diabetes24); however, it is not sufficient to say that a low Vit D level is the cause of the increased blood glucose. A meta-analysis comprising small-scale randomized controlled studies regarding Vit D supplementation (20 studies, n=2,703) showed that Vit D improved insulin resistance indicator (homeostasis model assessment-insulin resistance) but without reducing the level of glycated hemoglobin.25) Subsequently, a large-scale randomized controlled study was conducted to examine the preventive effect of Vit D on diabetes (D2d study, n=2,423). In the average 2.5-year follow-up monitoring, the supplementation of 4,000 IU of Vit D could not prevent the incidence of new-onset diabetes (risk ratio, 0.88 [95% CI, 0.75, 1.02]; p=0.12). The mean baseline Vit D level was 27.7 ng/mL, and most of the patients did not show Vit D deficiency. A post hoc analysis was carried out to investigate the individuals showing a Vit D level below 12 ng/mL, and the risk ratio for the incidence of new-onset diabetes was 0.38 (95% CI, 0.18, 0.80) for Vit D.26) Thus, it remains inconclusive whether Vit D has a preventive effect on diabetes in Vit D-deficient individuals.
Certain types of dairy products were shown to prevent the incidence of diabetes.27) In a metaanalysis comprising 7 cohort studies, common plain milk had no preventive effect (relative risk ratio, 0.95 after correction [95% CI, 0.86, 1.05]), while fermented yogurts showed the most significant effect on diabetes prevention (relative risk ratio, 0.83 [95% CI, 0.74, 0.90]). Dairy products with low fat content showed higher preventive effects than those with high fat content (relative risk ratio, 0.82 vs. 1.00).28) However, many commercially available fermented yogurts contain simple sugars.
Coffee intake was consistently shown to reduce the risk of the incidence of new-onset diabetes.29) A meta-analysis comprising 28 prospective cohort studies (n=1,109,272) also showed that coffee intake could reduce the risk of diabetes in direct proportion to the amount of intake. The individuals who drank 6 cups of coffee a day showed 33% lower risk of diabetes than the non-drinkers (relative risk ratio, 0.67 [0.61–0.74]). Considering that decaffeinated coffee also has a similar preventive effect on diabetes, a bioactive compound other than caffeine is thought to be responsible for the preventive effect.
In a meta-analysis comprising 18 prospective cohort studies, the intake of nuts was reported, without correction, to reduce the risk of T2D by 20% (relative risk ratio, 0.80 [95% CI, 0.69, 0.94]); however, after correcting for body mass index (BMI), no significant difference was found (relative risk ratio, 1.03 [95% CI, 0.91, 1.16]).30) Among the different types of nuts, walnuts showed higher preventive effect on T2D than peanuts.31) A meta-analysis comprising 2 cohort studies (n=137,956) showed that compared with the control group, the group with 2 or more intake of walnuts per week showed 33% lower risk of T2D (relative risk ratio, 0.62–0.94; n=137,956), for which the significance remained valid even after correcting for BMI (relative risk ratio, 0.76 [0.62–0.94]). In this study, the effect of reducing the risk of T2D was proportional to the level of walnut intake. Despite the high fat and high calorie contents of nuts, the intake showed a correlation with weight loss rather than with weight gain.
The main etiological factors for T2D are the increase in insulin resistance and the decrease in insulin secretion. Numerous studies have attempted to prevent T2D using various drugs that lead to increased insulin secretion or improved insulin resistance. In the aforementioned DPP, the efficacy of metformin, a type of oral hypoglycemic agent, in preventing the progression to T2D was determined as part of the investigation.4) The administration of metformin was found to be effective particularly in young obese patients, as the incidence of diabetes significantly decreased in the group of patients aged 25–40 years and in the group of patients with BMI ≥36 kg/m2. Moreover, compared with lifestyle modification, the administration of metformin was cost-effective.32-34)
In a study involving Caucasian individuals, thiazolidinedione showed a good diabetes preventive effect. In the Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication, treatment with rosiglitazone led to a 60% decrease in the incidence of diabetes. On the contrary, the incidence of heart failure significantly increased.35) In the Actos Now for the prevention of diabetes (ACT NOW) study, the 2.4-year follow-up monitoring of 602 patients with impaired glucose tolerance treated with 45 mg of pioglitazone showed that the incidence of diabetes was reduced by 72% in this group.36) After a drug washout period of 1 year, the incidence of new-onset diabetes in the pioglitazone administration group was low (56%).37) However, it remains uncertain whether results such as those of the “diabetes preventive effect of thiazolidinedione in Caucasian patients” can be obtained from Korean individuals with a relatively low percentage of insulin resistance.
The Study to Prevent Non-Insulin-Dependent Diabetes Mellitus Trial was conducted to examine whether acarbose could prevent the progression to T2D in patients diagnosed with impaired glucose tolerance.38) Among the 1,429 patients included in this study, the treatment group showed a 25% delayed progression to diabetes compared with the placebo group. However, the acarbose administration group showed a 30% dropout rate due to a gastrointestinal side effect, whereas the control group showed an 18% dropout rate. Another agent called voglibose showed similar effects. In the Voglibose Ph-3 study, the incidence of diabetes was found to have decreased by 40% compared with that of the control group, based on the results of the 4-year follow-up monitoring of 1,429 patients with impaired glucose tolerance.39)
The Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research (NAVIGATOR) investigated the effects of nateglinide, an insulin secretagogue, as a prospective, double-blind study involving 9,306 patients with impaired glucose tolerance and applied a 2×2 random allocation (valsartan or placebo, nateglinide, or placebo).40) The target patients were either those aged ≥55 years with one or more cardiovascular risk factors or those aged ≥50 years with a cardiovascular disease. The 5-year follow-up monitoring showed that treatment with nateglinide did not prevent the incidence of diabetes or cardiovascular disease compared with the control.
In the Outcome Reduction With Initial Glargine Intervention (ORIGIN) study, the 6.2-year follow-up monitoring of 1,456 non-diabetic individuals (737 in the glargine group; 719 in the standard care group) showed that the incidence of new-onset diabetes was 28% lower in the insulin glargine group (24.7% in the glargine group and 31.2% in the standard care group; risk ratio, 0.72 [95% CI, 0.58, 0.91; p=0.006]).41) After terminating the ORIGIN study, the 2.7-year follow-up study (ORIGIN and Legacy Effects) showed that the difference in the level of glycated hemoglobin during the study period was maintained after the study (6.6% in the glargine group and 6.7% in the standard care group; p=0.025).42) However, as weight gain was observed in the glargine group with the incidence of hypoglycemia, the administration of insulin glargine cannot yet be recommended to prediabetic patients for diabetes prevention.
Despite the limitation of being a post-hoc analysis based on the secondary endpoint, the SCALE study followed up 2,254 patients with either BMI >30 kg/m2 or BMI ≥27 kg/m2 and a complication for 3 years, dividing the patients into the placebo group (n=749) and the group administered with 3.0 mg of liraglutide (n=1,505). The groups showed diabetes incidence rates of 6.23% and 1.76%, respectively.43) The risk of diabetes incidence decreased by 79% (95% CI, 0.13, 0.34) after treatment with liraglutide. The group that received liraglutide treatment showed a weight loss of 6.1%, while the placebo group showed a weight loss of 1.9% (with an intergroup difference of −4.3%; 95% CI, −4.0, −3.7; p<0.001). However, as the study was not based on the incidence of diabetes as the primary endpoint, the criteria for determining the incidence of diabetes in the study patients were inconsistent. For most accurate evaluation, glycated hemoglobin and oral glucose tolerance tests should be performed after a certain period of drug washout. Second, a test to verify whether the observed effects are maintained after a drug washout is necessary. The ACT NOW study on pioglitazone showed that the diabetes preventive effect was maintained throughout the 1-year follow-up monitoring after terminating the study.36) Third, a 50% dropout rate is high and was considered as a limitation of the study. Despite the limitations, the 79% diabetes preventive effect of liraglutide in this study, which led to a 4.7% weight loss in patients, is considered as a good level in terms of the reduced risk of diabetes based on the percentage of weight loss. Although a direct comparison is difficult, the risk of diabetes was reduced by 40–50% in the Finnish Diabetes Prevention Study that led to a 5–7% weight loss after implementing the lifestyle intervention. The value reported after treatment with liraglutide is comparable to the 72% reduced risk reported after treatment with pioglitazone in the ACT NOW study where an oral agent showed the most outstanding diabetes preventive effect thus far.
A significant number of drugs, called diabetes medications, are effective in preventing the occurrence of diabetes are. Thus, the use of diabetes medications for prevention is considered as an early phase of diabetes treatment. A drug should have minimum side effects, proven long-term effect, and relatively low cost to be used for diabetes prevention in clinical practice. In addition, the effect of the drug should last for a reliable period even after drug washout, ultimately proving the delayed incidence of diabetes. Thus far, no drug has met all the aforementioned criteria. In the current guideline, metformin is the drug recommended for the high-risk group. However, the administration of metformin to prediabetic patients remains challenging in clinical practice, as it is not included in the Ministry of Food and Drug Safety drug indications; thus, only the uninsured benefit is available. To date, no other drug is indicated for prediabetic patients. Liraglutide (3 mg/pen) is an out-of-pocket obesity medication that can be administered without a concern for the uninsured benefit.
Recently, a number of studies conducted in patients receiving active drug therapy for cardiovascular diseases have reported a significant decrease in the incidence of T2D during the study period. The Heart Outcome Prevention Evaluation investigated whether ramipril had a diabetes preventive effect in the high-risk group. The study was conducted in 5,720 individuals aged ≥55 years and with a vascular disease but no history of diabetes. The mean follow-up period was 4.5 years, and the participants were divided into the placebo group (2,883 individuals) and the ramipril group (10 mg/day of ramipril administration; 2,837 individuals). The incidence rates of diabetes were 5.4% and 3.6%, respectively, while the incidence was shown to have decreased by 34%.44)
In the NAVIGATOR study, the cumulative incidence of diabetes as the primary endpoint after 5 years was 33.1% in the group administered with valsartan, showing a 14% reduced incidence of T2D compared with the 36.8% in the placebo group, with a significant difference (hazard ratio, 0.86; p<0.001).45) The effect of valsartan in preventing T2D was consistently observed in subgroup analysis.
According to a recent meta-analysis of angiotensin II receptor blockers (ARB drugs) and the incidence of new-onset diabetes (on 11 randomized controlled studies), ARB drugs showed a significant effect on the prevention of T2D. The reduction rate was 17% compared with the control (relative risk ratio, 0.83 [0.78–0.89]), 27% compared with beta-blockers (relative risk ratio, 0.73 [0.62–0.87]), 24% compared with calcium antagonists (relative risk ratio, 0.76 [0.68–0.85]), and 43% compared with non-ARB drugs (relative risk ratio, 0.57 [0.36–0.91]).46)
The XENical in the prevention of diabetes in obese subjects study investigated the incidence of T2D after dividing 3,305 patients with BMI ≥30 kg/m2 into the orlistat administration group and the control group. As a gastrointestinal lipase inhibitor, orlistat reduces the dietary fat absorption by 30% to induce weight loss. The orlistat administration group showed an average weight loss of 6.9 kg in year 4, while the placebo group showed a weight loss of 4.1%, which indicated the change in lifestyle. In the orlistat administration group, the incidence of T2D was reduced by approximately 37%.47)
In the Behavioral Modification and Lorcaserin for Overweight and Obesity Management trial, a phase III clinical study that investigated the effect of lorcaserin in a 52-week period, and in the post hoc analysis of the Behavioral Modification and Lorcaserin Second Study for Overweight and Obesity Management trial (n=6,136), the incidence of new-onset diabetes was lower in the lorcaserin administration group (lorcaserin, 3.2%; placebo, 5.0%; p=0.032).48)
In the 2-year follow-up monitoring the administration of phentermine/topiramate extendedrelease, the groups administered with 7.5/46 mg and 15/92 mg demonstrated 70.5% and 78.7% reduction in diabetes incidence, respectively, compared with the control group (p<0.05).49)
A longitudinal study was conducted in 136 patients with impaired glucose tolerance and severe obesity. No surgical intervention was performed in the control group (27 patients), while the remaining 109 patients underwent bariatric surgery. In the intervention group, a 22.5-kg weight loss was achieved over the course of 4–6 years, and 1 out of 109 patients developed diabetes, indicating the incidence of 0.15/100 persons/year. On the contrary, the incidence was 4.72/100 persons/year in the control group, which implied a 30-fold decrease in the diabetes incidence through bariatric surgery (relative risk ratio, 0.83; p<0.001).50)
Presently, the American Diabetes Association recommends a 7% weight loss in prediabetic patients for diabetes prevention. In Korea, many prediabetic patients are lean; hence, the 7% weight loss cannot be applied to all patients. Koreans differ from Caucasians in terms of the pathophysiology of T2D. In the study conducted in Korea, the compensatory hypersecretion of insulin in response to the increase in insulin resistance was not observed among prediabetic patients. Compared with Caucasians, the percentage of obese Korean patients is lesser, while numerous studies have reported that insulin deficiency is commonly observed as early as at the time of diagnosis. Therefore, it is difficult to directly apply the findings of various studies regarding the diabetes prevention in Caucasian patients to Asian patients. The Korean Diabetes Prevention Study is an ongoing national research conducted by the Ministry of Health and Welfare. The study was launched in April 2016, involving multiple institutions (university hospitals and public health centers) and targeting the high-risk group. Thus far, the intermediate results have been published; the average 18-month follow-up monitoring in the first research project conducted in a university hospital showed that the cumulative incidence of diabetes was 37.6% lower in the lifestyle intervention group (14.1%) than in the standard care group (22.7%). The diabetes incidence in the metformin administration group was 16.9%, which was lower than that of the standard care group but without a significant difference. In the second research project conducted in public health centers, the average 12-month followup monitoring found that the cumulative incidence of diabetes was 54.7% lower in the lifestyle intervention group (2.4%) than in the standard care group (5.3%).
In most countries, the prevention of diabetes has become the most pressing issue, i.e., to stop an exponential increase in diabetes incidence. At present, the best treatment for diabetes is prevention, as diabetes often accompanies a complication at the time of diagnosis; despite active glycemic control, the incidence or progression of a complication cannot be completely prevented. The diabetes preventive effect of lifestyle modification has been verified in multiple studies, with an additional benefit of cost-effectiveness (Figure 1). Long-term follow-up monitoring studies have also shown that the initial preventive effect was maintained after terminating the study. Hence, screening must be performed in the high-risk group, followed by education on active lifestyle modification. The administration of metformin for diabetes prevention may be considered in obese patients aged <60 years with a history of gestational diabetes. As in the case of diabetes patients, prediabetes patients also show an increased risk of arteriosclerotic cardiovascular disease. To reduce the incidence of arteriosclerotic cardiovascular disease, evaluation of the cardiovascular disease risk factors should be conducted along with the lifestyle interventions.
REFERENCES
1. Lindström J, Louheranta A, Mannelin M, Rastas M, Salminen V, Eriksson J, Uusitupa M, Tuomilehto J; Finnish Diabetes Prevention Study Group. The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care. 2003; 26:3230–6.
2. Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard BV. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997; 20:537–44.

3. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002; 346:393–403.

4. Williamson DF, Vinicor F, Bowman BA; Centers for Disease Control and Prevention Primary Prevention Working Group. Primary prevention of type 2 diabetes mellitus by lifestyle intervention: implications for health policy. Ann Intern Med. 2004; 140:951–7.

5. Diabetes Prevention Program Research Group, Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, Brown-Friday JO, Goldberg R, Venditti E, Nathan DM. 10-year followup of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009; 374:1677–86.

6. Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015; 3:866–75.
7. Lindström J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemiö K, Hämäläinen H, Härkönen P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Mannelin M, Paturi M, Sundvall J, Valle TT, Uusitupa M, Tuomilehto J; Finnish Diabetes Prevention Study Group. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006; 368:1673–9.

8. Lindström J, Peltonen M, Eriksson JG, Ilanne-Parikka P, Aunola S, Keinänen-Kiukaanniemi S, Uusitupa M, Tuomilehto J; Finnish Diabetes Prevention Study (DPS). Improved lifestyle and decreased diabetes risk over 13 years: long-term follow-up of the randomised Finnish Diabetes Prevention Study (DPS). Diabetologia. 2013; 56:284–93.

9. Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, Li H, Li H, Jiang Y, An Y, Shuai Y, Zhang B, Zhang J, Thompson TJ, Gerzoff RB, Roglic G, Hu Y, Bennett PH. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet. 2008; 371:1783–9.

10. Li G, Zhang P, Wang J, An Y, Gong Q, Gregg EW, Yang W, Zhang B, Shuai Y, Hong J, Engelgau MM, Li H, Roglic G, Hu Y, Bennett PH. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol. 2014; 2:474–80.

11. Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet. 2014; 383:1999–2007.

12. Hemmingsen B, Gimenez-Perez G, Mauricio D, Roqué I Figuls M, Metzendorf MI, Richter B. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst Rev. 2017; 12:CD003054.

13. Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016; 133:187–225.

14. Parker AR, Byham-Gray L, Denmark R, Winkle PJ. The effect of medical nutrition therapy by a registered dietitian nutritionist in patients with prediabetes participating in a randomized controlled clinical research trial. J Acad Nutr Diet. 2014; 114:1739–48.

15. Jacobs S, Harmon BE, Boushey CJ, Morimoto Y, Wilkens LR, Le Marchand L, Kröger J, Schulze MB, Kolonel LN, Maskarinec G. A priori-defined diet quality indexes and risk of type 2 diabetes: the Multiethnic Cohort. Diabetologia. 2015; 58:98–112.

16. Salas-Salvadó J; Guasch-Ferré M, Lee CH, Estruch R, Clish CB, Ros E. Protective effects of the Mediterranean diet on type 2 diabetes and metabolic syndrome. J Nutr. 2015; 146:920S–7S.
17. Salas-Salvadó J, Bulló M, Babio N, Martínez-González MÁ, Ibarrola-Jurado N, Basora J, Estruch R, Covas MI, Corella D, Arós F, Ruiz-Gutiérrez V, Ros E; PREDIMED Study Investigators. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care. 2011; 34:14–9.
18. Koloverou E, Esposito K, Giugliano D, Panagiotakos D. The effect of Mediterranean diet on the development of type 2 diabetes mellitus: a meta-analysis of 10 prospective studies and 136,846 participants. Metabolism. 2014; 63:903–11.

19. Wu JH, Micha R, Imamura F, Pan A, Biggs ML, Ajaz O, Djousse L, Hu FB, Mozaffarian D. Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Br J Nutr. 2012; 107 Suppl 2:S214–27.

20. Muley A, Muley P, Shah M. ALA, fatty fish or marine n-3 fatty acids for preventing DM?: a systematic review and meta-analysis. Curr Diabetes Rev. 2014; 10:158–65.

21. Muraki I, Imamura F, Manson JE, Hu FB, Willett WC, van Dam RM, Sun Q. Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. BMJ. 2013; 347:f5001.

22. Mursu J, Virtanen JK, Tuomainen TP, Nurmi T, Voutilainen S. Intake of fruit, berries, and vegetables and risk of type 2 diabetes in Finnish men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr. 2014; 99:328–33.

23. Cooper AJ, Forouhi NG, Ye Z, Buijsse B, Arriola L, Balkau B, Barricarte A, Beulens JW, Boeing H, Büchner FL, Dahm CC, de Lauzon-Guillain B, Fagherazzi G, Franks PW, Gonzalez C, Grioni S, Kaaks R, Key TJ, Masala G, Navarro C, Nilsson P, Overvad K, Panico S, Ramón Quirós J, Rolandsson O, Roswall N, Sacerdote C, Sánchez MJ, Slimani N, Sluijs I, Spijkerman AM, Teucher B, Tjonneland A, Tumino R, Sharp SJ, Langenberg C, Feskens EJ, Riboli E, Wareham NJ, InterAct Consortium. Fruit and vegetable intake and type 2 diabetes: EPIC-InterAct prospective study and meta-analysis. Eur J Clin Nutr. 2012; 66:1082–92.

24. Song Y, Wang L, Pittas AG, Del Gobbo LC, Zhang C, Manson JE, Hu FB. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2013; 36:1422–8.
25. Li X, Liu Y, Zheng Y, Wang P, Zhang Y. The effect of vitamin D supplementation on glycemic control in type 2 diabetes patients: a systematic review and meta-analysis. Nutrients. 2018; 10:375.

26. Pittas AG, Dawson-Hughes B, Sheehan P, Ware JH, Knowler WC, Aroda VR, Brodsky I, Ceglia L, Chadha C, Chatterjee R, Desouza C, Dolor R, Foreyt J, Fuss P, Ghazi A, Hsia DS, Johnson KC, Kashyap SR, Kim S, LeBlanc ES, Lewis MR, Liao E, Neff LM, Nelson J, O'Neil P, Park J, Peters A, Phillips LS, Pratley R, Raskin P, Rasouli N, Robbins D, Rosen C, Vickery EM, Staten M, D2d Research Group. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019; 381:520–30.

27. Tong X, Dong JY, Wu ZW, Li W, Qin LQ. Dairy consumption and risk of type 2 diabetes mellitus: a metaanalysis of cohort studies. Eur J Clin Nutr. 2011; 65:1027–31.

28. Chen M, Sun Q, Giovannucci E, Mozaffarian D, Manson JE, Willett WC, Hu FB. Dairy consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. BMC Med. 2014; 12:215.

29. Ding M, Bhupathiraju SN, Chen M, van Dam RM, Hu FB. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: a systematic review and a dose-response meta-analysis. Diabetes Care. 2014; 37:569–86.

30. Jiang R, Manson JE, Stampfer MJ, Liu S, Willett WC, Hu FB. Nut and peanut butter consumption and risk of type 2 diabetes in women. JAMA. 2002; 288:2554–60.

31. Pan A, Sun Q, Manson JE, Willett WC, Hu FB. Walnut consumption is associated with lower risk of type 2 diabetes in women. J Nutr. 2013; 143:512–8.

32. Diabetes Prevention Program Research Group. The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care. 2012; 35:723–30.
33. Herman WH, Hoerger TJ, Brandle M, Hicks K, Sorensen S, Zhang P, Hamman RF, Ackermann RT, Engelgau MM, Ratner RE; Diabetes Prevention Program Research Group. The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med. 2005; 142:323–32.

34. Herman WH. The cost-effectiveness of diabetes prevention: results from the Diabetes Prevention Program and the Diabetes Prevention Program Outcomes Study. Clin Diabetes Endocrinol. 2015; 1:9.

35. DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators, Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman RR. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006; 368:1096–105.

36. DeFronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, Clement SC, Henry RR, Hodis HN, Kitabchi AE, Mack WJ, Mudaliar S, Ratner RE, Williams K, Stentz FB, Musi N, Reaven PD; ACT NOW Study. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. 2011; 364:1104–15.

37. Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, Clement SC, Henry RR, Kitabchi AE, Mudaliar S, Ratner RE, Stentz FB, Musi N, Reaven PD, DeFronzo RA. Diabetes Incidence and Glucose Tolerance after Termination of Pioglitazone Therapy: Results from ACT NOW. J Clin Endocrinol Metab. 2016; 101:2056–62.

38. Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M; STOP-NIDDM Trail Research Group. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002; 359:2072–7.

39. Kawamori R, Tajima N, Iwamoto Y, Kashiwagi A, Shimamoto K, Kaku K; Voglibose Ph-3 Study Group. Voglibose for prevention of type 2 diabetes mellitus: a randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet. 2009; 373:1607–14.

40. NAVIGATOR Study GroupHolman RR, Haffner SM, McMurray JJ, Bethel MA, Holzhauer B, Hua TA, Belenkov Y, Boolell M, Buse JB, Buckley BM, Chacra AR, Chiang FT, Charbonnel B, Chow CC, Davies MJ, Deedwania P, Diem P, Einhorn D, Fonseca V, Fulcher GR, Gaciong Z, Gaztambide S, Giles T, Horton E, Ilkova H, Jenssen T, Kahn SE, Krum H, Laakso M, Leiter LA, Levitt NS, Mareev V, Martinez F, Masson C, Mazzone T, Meaney E, Nesto R, Pan C, Prager R, Raptis SA, Rutten GE, Sandstroem H, Schaper F, Scheen A, Schmitz O, Sinay I, Soska V, Stender S, Tamás G, Tognoni G, Tuomilehto J, Villamil AS, Vozár J, Califf RM. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010; 362:1463–76.
41. ORIGIN Trial Investigators, Gerstein HC, Bosch J, Dagenais GR, Díaz R, Jung H, Maggioni AP, Pogue J, Probstfield J, Ramachandran A, Riddle MC, Rydén LE, Yusuf S. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012; 367:319–28.

42. ORIGIN Trial Investigators. Cardiovascular and other outcomes postintervention with insulin glargine and omega-3 fatty acids (ORIGINALE). Diabetes Care. 2016; 39:709–16.
43. le Roux CW, Astrup A, Fujioka K, Greenway F, Lau DC, Van Gaal L, Ortiz RV, Wilding JP, Skjøth TV, Manning LS, Pi-Sunyer X; SCALE Obesity Prediabetes NN8022-1839 Study Group. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in Individuals with prediabetes: a randomised, doubleblind trial. Lancet. 2017; 389:1399–409.
44. Yusuf S, Gerstein H, Hoogwerf B, Pogue J, Bosch J, Wolffenbuttel BH, Zinman B; HOPE Study Investigators. Ramipril and the development of diabetes. JAMA. 2001; 286:1882–5.

45. NAVIGATOR Study Group, McMurray JJ, Holman RR, Haffner SM, Bethel MA, Holzhauer B, Hua TA, Belenkov Y, Boolell M, Buse JB, Buckley BM, Chacra AR, Chiang FT, Charbonnel B, Chow CC, Davies MJ, Deedwania P, Diem P, Einhorn D, Fonseca V, Fulcher GR, Gaciong Z, Gaztambide S, Giles T, Horton E, Ilkova H, Jenssen T, Kahn SE, Krum H, Laakso M, Leiter LA, Levitt NS, Mareev V, Martinez F, Masson C, Mazzone T, Meaney E, Nesto R, Pan C, Prager R, Raptis SA, Rutten GE, Sandstroem H, Schaper F, Scheen A, Schmitz O, Sinay I, Soska V, Stender S, Tamás G, Tognoni G, Tuomilehto J, Villamil AS, Vozár J, Califf RM. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010; 362:1477–90.
46. Geng DF, Jin DM, Wu W, Xu Y, Wang JF. Angiotensin receptor blockers for prevention of new-onset type 2 diabetes: a meta-analysis of 59,862 patients. Int J Cardiol. 2012; 155:236–42.

47. Torgerson JS, Hauptman J, Boldrin MN; Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004; 27:155–61.
48. Nesto R, Fain R, Li Y, Shanahan W. Evaluation of lorcaserin on progression of prediabetes to type 2 diabetes and reversion to euglycemia. Postgrad Med. 2016; 128:364–70.

49. Garvey WT, Ryan DH, Henry R, Bohannon NJ, Toplak H, Schwiers M, Troupin B, Day WW. Prevention of type 2 diabetes in subjects with prediabetes and metabolic syndrome treated with phentermine and topiramate extended release. Diabetes Care. 2014; 37:912–21.

50. Carlsson LM, Peltonen M, Ahlin S, Anveden Å, Bouchard C, Carlsson B, Jacobson P, Lönroth H, Maglio C, Näslund I, Pirazzi C, Romeo S, Sjöholm K; Sjöström E, Wedel H, Svensson PA; Sjöström L. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med. 2012; 367:695–704.

Figure 1.
Summary of risk reduction of incident type 2 diabetes during clinical trials.
DPP = Diabetes Prevention Program; DPS = Diabetes Prevention Study.
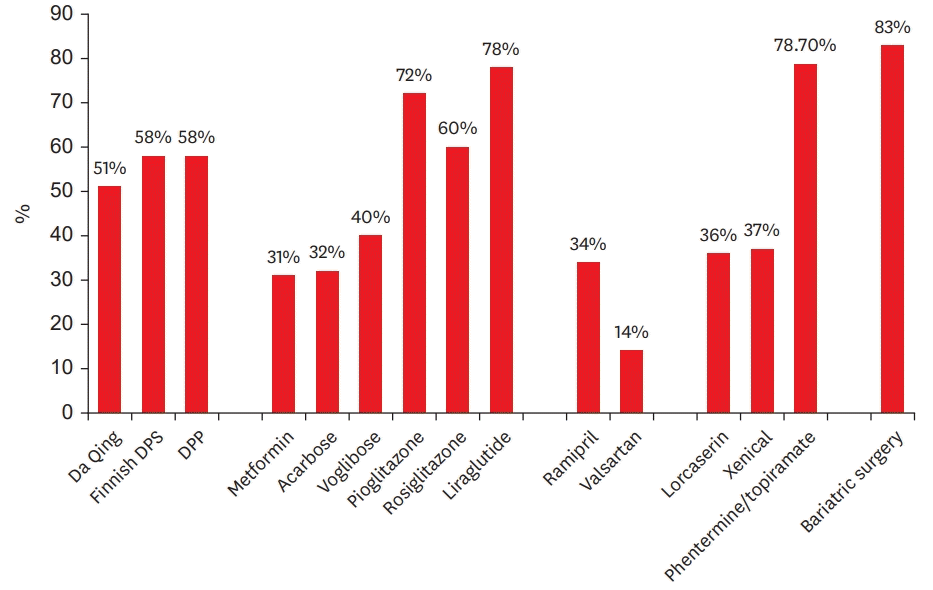
Table 1.
Risk factors of type 2 diabetes




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download