INTRODUCTION
METHODS
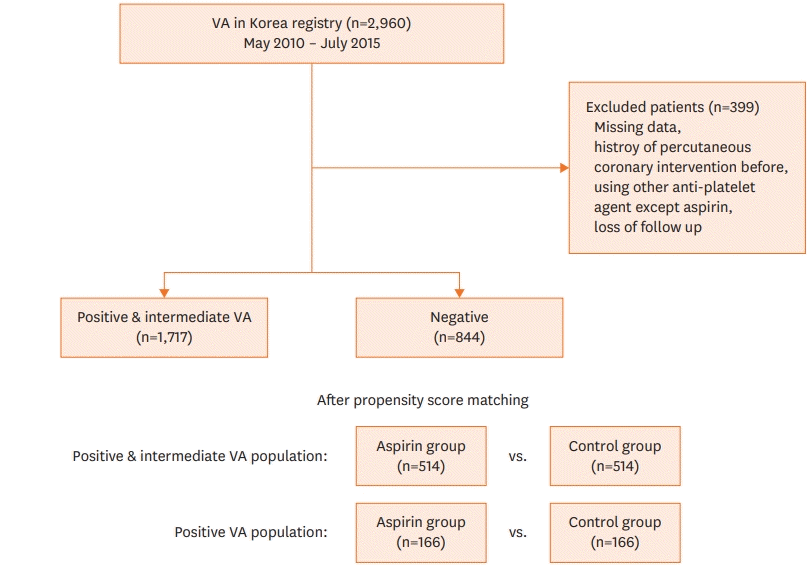
Study population
CAG and provocation test: definitions of positive and intermediate results
Definition and primary outcome
Follow-up
Statistical analysis
RESULTS
Patient enrollment and incidence
Baseline characteristics
Table 1.
Values are presented as means±standard deviation or number (%). Aspirin denotes 100 mg/day aspirin.
ACEI = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; BMI = body mass index; CCB = calcium channel blocker; COPD = chronic obstructive pulmonary disease; HDL-C = high density lipoprotein cholesterol; LDL-C = low density lipoprotein cholesterol; LVEF = left ventricle ejection fraction; SMD = standardized mean difference. TC = total cholesterol.
Clinical outcomes of the overall population
Clinical outcomes of the PS-matched group analysis
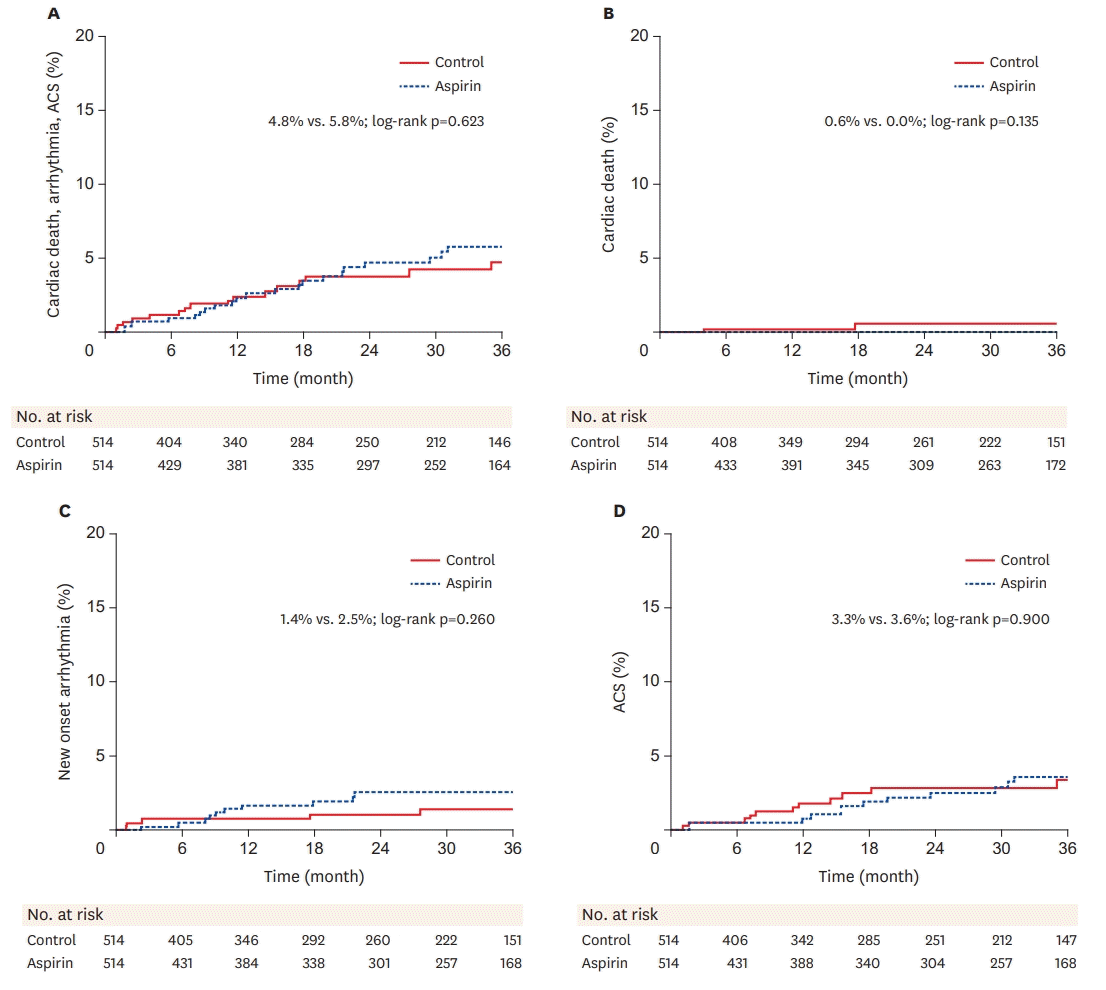 | Figure 2.Kaplan-Meier incidence curves of outcomes in 514 propensity-matched pairs in the positive and intermediate vasospastic angina groups. Long-term incidences in the 2 groups are presented as percentages and were statistically compared with the log-rank test. (A) Incidences of composite major adverse cardiac event, (B) Incidences of cardiac death, (C) Incidences of arrhythmia, (D) Incidences of acute coronary syndrome.
ACS = acute coronary syndrome.
|
Table 2.
Values are presented as number (%). HRs are for the aspirin group as compared to the control group.
ACS = acute coronary syndrome; AV = atrioventricular; CD = cardiac death; CI = confidence interval; ED = emergency department; HR = hazard ratio; MACE = major adverse cardiac event; NSTEMI = non-ST elevation myocardial infarction; STEMI = ST elevation myocardial infarction; VA = vasospastic angina; VF = ventricular fibrillation; VT = ventricular tachycardia.
Cox regression hazards analysis of composite MACE in PS-matched patients
Subgroup analysis among PS-matched patients
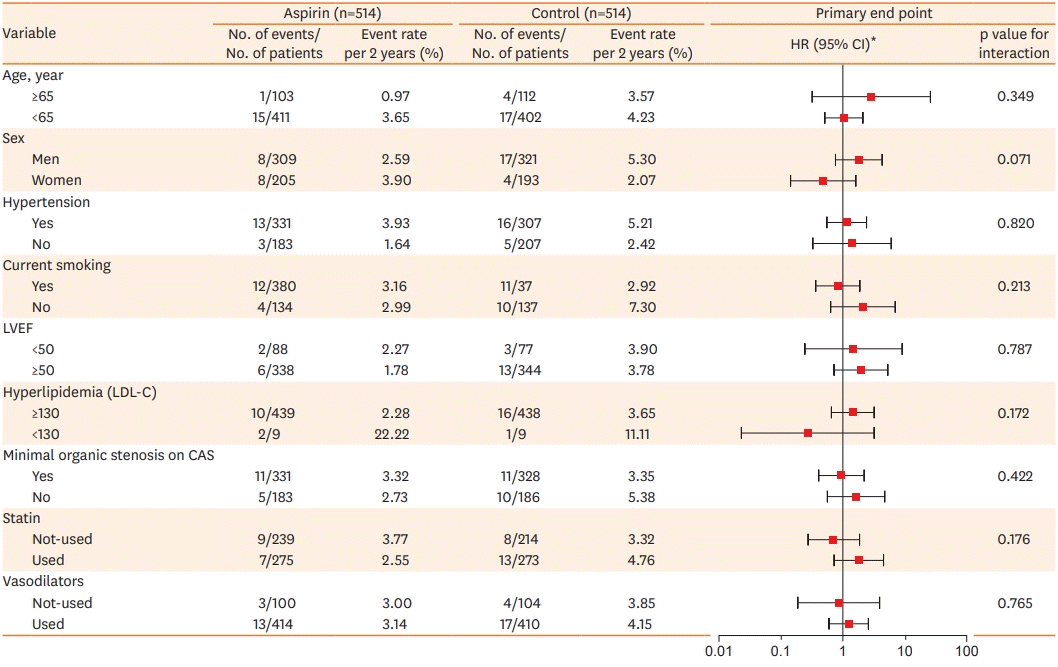 | Figure 3.HR for aspirin vs. control group and event rates for the primary composite outcome among propensity-matched patient.
CAG = coronary angiogram; CI = confidence interval; HR = hazard ratio; LAS = low dose aspirin; LDL-C = Low-density lipoprotein Cholesterol; LVEF = left ventricle ejection fraction.
*HRs are for the LAS group, as compared control group.
|
Sensitivity analysis of a VA-positive population
DISCUSSION
Table 3.
| Characteristics |
Present cohort |
Ishii et al.21) |
Lim et al.14) |
||||
|---|---|---|---|---|---|---|---|
| No aspirin (n=514) | Aspirin (n=514) | No aspirin (n=112) | Aspirin (n=112) | No aspirin (n=287) | Aspirin (n=434) | ||
| Enrollment | 2010–2015 | 1991–2010 | 2003–2014 | ||||
| Registry information | 11 multi-centers, prospective | Single center, retrospective | Single center, retrospective | ||||
| Race | Korean | Japanese | Korean | ||||
| Follow duration (yr) | 2.00 (0.86–3.01) | 5 | 4.34 | ||||
| Provocation drug | Ergonovine | Acetylcholine | Ergonovine | ||||
| Age (yr) | 55.94±10.75 | 55.55±10.93 | 67.0±8.4 | 66.0±9.5 | 55.0 (49.0–62.5) | 56.0 (49.0–62.0) | |
| Male | 309 (60.1) | 321 (62.5) | 65 (58.0) | 65 (58.0) | 243 (84.7) | 359 (82.7) | |
| Hypertension | 183 (35.6) | 207 (40.3) | 57 (50.9) | 52 (46.4) | 104 (36.2) | 156 (36.0) | |
| Diabetes mellitus | 43 (8.4) | 49 (9.5) | 27 (24.1) | 26 (23.2) | 66 (23.0) | 98 (22.6) | |
| Current Smoking | 134 (26.1) | 137 (26.7) | 52 (46.4) | 59 (52.7) | 87 (30.3) | 127 (29.3) | |
| Dyslipidemia | 93 (18.1) | 75 (14.6) | 60 (53.6) | 62 (55.4) | 62 (21.6) | 91 (21.0) | |
| BMI (kg/m2) | 24.62±3.15 | 24.82±3.02 | 23.7 ± 3.9 | 24.0 ± 3.1 | 24.7 (22.7–26.3) | 24.4 (22.8–26.0) | |
| Laboratory findings | |||||||
| Total cholesterol (mg/dL) | 176.29±37.31 | 173.38±35.66 | 190.3±34.2 | 188.8±31.7 | 171 (150–193) | 169 (150–197) | |
| Triglyceride (mg/dL) | 142.01±88.10 | 140.99±108.51 | 121.8±64.0 | 133.0±76.2 | 129 (88–191) | 128 (91–196) | |
| LDL (ml/dL) | 104.55±32.20 | 103.95±30.65 | 111.5±30.7 | 110.9±27.7 | 106 (86–123) | 105 (86–128) | |
| HDL (mg/dL) | 46.76±12.50 | 47.29±13.93 | 54.4±16.3 | 51.3±16.9 | 46 (39–55) | 45 (39–53) | |
| LVEF (%) | 64.93±6.56 | 64.37±6.63 | - | - | 64.0 (60.0–68.0) | 65.0 (61.0–68.0) | |
| CAG findings | |||||||
| Minimal organic stenosis | 183 (35.6) | 186 (36.2) | - | - | 86 (30.0) | 143 (32.9) | |
| Medication at discharge | |||||||
| Vasodilators | 414 (80.5) | 410 (79.8) | 23 (19.7) | 27 (24.1) | 177 (61.7) | 267 (61.5) | |
| CCB | 470 (91.4) | 460 (89.5) | 101 (90.2) | 104 (92.9) | 275 (95.8) | 420 (96.9) | |
| Statin | 275 (53.5) | 273 (53.1) | 40 (35.7) | 38 (33.9) | 113 (39.4) | 182 (42.0) | |
| ACEI/ARB | 81 (15.8) | 89 (17.3) | 25 (22.3) | 33 (29.5) | 43 (15.0) | 69 (15.9) | |
| Beta blocker | 22 (4.3) | 26 (5.1) | 7 (6.3) | 6 (5.4) | 0 (0.0) | 1 (0.2) | |
| Clinical outcome | |||||||
| All-cause of death | 2 (0.4) | 1 (0.2) | - | - | 9 (2.8) | 10 (2.2) | |
| Cardiac death | 2 (0.4) | 0 (0.0) | 0 (0) | 2 (1.8) | 3 (0.9) | 4 (0.9) | |
| Arrhythmia | 5 (1.0) | 10 (1.9) | - | - | - | - | |
| Myocardial infarction | 1 (0.2) | 0 (0.0) | 0 (0) | 0 (0) | 2 (0.6) | 9 (2.0) | |
| Unstable angina | 10 (1.9) | 12 (2.3) | 6 (5.4) | 2 (1.8) | - | - | |
| Rehospitalization/ED visit | 69 (13.4) | 78 (15.2) | - | - | 36 (11.2) | 94 (20.6) | |
Values are presented as means±standard deviation or number (%). HRs are for the LAS group, as compared control group.
ACEI = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; BMI = body mass index; CAG = coronary angiogram; CCB = calcium channel blocker; ED = emergency department; HDL = high density lipoprotein; HR = hazard ratio; LAS = lung allocation score; LDL = low density lipoprotein; LVEF = left ventricle ejection fraction; VA = vasospastic angina.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download