Abstract
Purpose
Oral sulfate tablets are abundantly used for bowel preparation before colonoscopy. However, their efficiency and safety for bowel preparation before colorectal surgery remain ill-defined. Herein, we aimed to compare the surgical site infection rates and efficiency between oral sulfate tablets and sodium picosulfate.
Methods
We designed a prospective, randomized, phase 2 clinical trial. Patients with colorectal cancer aged 19–75 years who underwent elective bowel resection and anastomosis by minimally invasive surgery were administered oral sulfate tablets or sodium picosulfate. Eighty-three cases were analyzed from October 2020 to December 2021. Surgical site infection within 30 days after surgery was considered the primary endpoint. Postoperative morbidities, the degree of bowel cleansing, and tolerability were the secondary endpoints.
Results
Surgical site infection was detected in 1 patient (2.5%) in the oral sulfate tablet group and 2 patients (4.7%) in the sodium picosulfate group, indicating no significant difference between the 2 groups. Postoperative morbidity and the degree of bowel cleansing bore no statistically significant differences. Furthermore, none of the investigated tolerability criteria, namely bloating, pain, nausea, vomiting, and discomfort, differed significantly between the 2 groups. The patients’ willingness to reuse the drug was also not significantly different between the 2 groups.
Notable effort has been put into preventing infection complications after colorectal surgery. This is largely implemented by decreasing the colonic content and intestinal microorganismal load through appropriate bowel preparation before surgery. In 1972, using a combination of mechanical bowel preparation and oral antibiotics, a method still in use [123], Nichols et al. [1] reported a reduction in the surgical site infection rate from 43% to 9%. While other studies have confirmed similar reductions in infection and anastomotic leakage rates [23], whether infection complications in colorectal surgery are consequently reduced remains controversial.
Many hospitals in Korea combine mechanical bowel preparation and oral antibiotics for elective colorectal cancer surgery [4]. However, patients often complain about the inconvenience of bowel preparation. In a previous study, 41% of the patients who underwent mechanical bowel preparation complained of nausea, vomiting, and abdominal pain [5], of whom 8% had to discontinue bowel lavage. Only 12% of those patients did not complain about discomfort. This is because polyethylene glycol (PEG) solutions, which have been used abundantly for bowel preparation since their introduction in 1980, have an unpleasant taste and a characteristic smell and are typically administered in great volumes. To resolve these problems, many alternative drugs have been developed. In a previous randomized trial, Kim et al. reported that low-volume oral picosulfate is more efficient in cleansing (91.5%) and better tolerated than the typical 4 L of PEG (81.1%) [6]. Currently, sodium picosulfate is used for bowel preparation before elective colorectal surgery in several hospitals, including the National Cancer Center.
Recently, oral sulfate tablets were developed and are currently widely used for bowel preparation before colonoscopy. Yang et al. [7] reported that oral sulfate tablets exhibited a bowel cleansing efficiency of 95.5%, similar to that by oral sodium sulfate (OSS; 98.2%), and were accompanied by fewer patient complaints. However, oral sulfate tablet has not yet been used for bowel preparation before colorectal surgery, and the surgical outcomes and patient compliance associated with its usage remain unknown. Regarding postoperative complications, surgical site infection rates with sodium picosulfate have been reported to amount to approximately 8% [8], but no studies have been conducted on oral sulfate tablets yet. We hypothesized that the use of oral sulfate tablets prior to elective colorectal cancer surgery would exhibit similar effects on surgical site infection as sodium picosulfate. To prove this, we compared the surgical site infection rates between patients undergoing bowel preparation with either agent. We also compared the postoperative morbidity, degree of bowel cleansing, and tolerability accompanying the administration of each of the 2 components.
This study was designed as a single-center, prospective, randomized, phase 2 clinical trial. As participants were not enrolled in other institutions, the study was conducted only at the National Cancer Center of Korea. All study procedures were conducted in accordance with the tenets of the Declaration of Helsinki. This study was registered with ClinicalTrials. gov (NCT04593446) and approved by the Institutional Review Board (IRB) of National Cancer Center (No. NCC 2020-0138). All participants provided written informed consent.
Patients with colorectal cancer aged 19–75 years who would undergo elective bowel resection and anastomosis by laparoscopic or robotic minimally invasive surgery were enrolled in the study. Patients who met the inclusion criteria were randomly administered oral sulfate tablets (ORA·FANG, Pharmbio Korea Co., Ltd., Seoul, Korea) or sodium picosulfate (Picosolution, Pharmbio Korea Co.) after they were made aware of the study protocol and provided informed consent.
Patients bearing the following characteristics were excluded from the study: (1) bowel obstruction due to a tumor; (2) familial adenomatous polyposis or inflammatory bowel disease; (3) pregnancy or breastfeeding; (4) history of an allergy to a bowel preparation agent; (5) severe renal impairment; (6) ascites; and (7) congestive heart failure.
A randomization table was generated using SAS version 9.4 (SAS Institute, Cary, NC, USA) in accordance with the block randomization method with stratification of the tumor location and block sizes of 2, 4, and 6. Patients were randomly assigned to experimental and control groups by an independent statistician with no clinical involvement in this trial. Throughout the trial, the surgeons who evaluated the degree of bowel cleansing during surgery were blinded to which group each patient was assigned.
Each patient in the oral sulfate tablet group was administered 14 tablets with 425 mL of water on the penultimate evening before surgery and subsequently drank 425 mL of water twice or more within 1 hour. The process was repeated the following morning (the last morning before surgery). In the sodium picosulfate group, the participants drank 1 bottle (170 mL) of sodium picosulfate solution on the penultimate evening before surgery and were recommended to consume 250 mL of water per hour thereafter. The procedure was repeated on the morning of the following day (the day before surgery).
Surgical site infections (primary endpoint) were assessed using the definitions published by the U.S. Centers for Disease Control and Prevention in 1999 [9]. They were classified as incision site infections or organ/space infections within 30 days after surgery. Incision site infections were defined as those of the skin, subcutaneous tissue, muscle, or fascia. Organ/space infections were defined as infections in all areas opened or manipulated during surgery except for those at the incision site. Surgical site infections were assessed by clinicians during the postoperative hospital stay or the first outpatient visit 3 weeks after surgery. Morbidities were evaluated up to 30 days after surgery based on the Clavien-Dindo classification. The degree of bowel cleansing was classified into 4 grades of remaining stool in the resected colon lumen during surgery (solid, soft, fluid, and none) and evaluated by the surgeon. The latter was unaware of which type of bowel preparation had been administered to the patient. Tolerability of bowel preparation was based on the patient’s answers to a questionnaire provided before surgery and involved their evaluation of bloating, pain, nausea, vomiting, and discomfort using the following 4 grades: 1, none; 2, mild; 3, significant; and 4, unbearable.
This study was originally designed to assess the noninferiority of oral sulfate tablets to sodium picosulfate in reducing surgical site infection rates. Previous studies suggested that the incidence of surgical site infections in patients with mechanical bowel preparation agents is 8% [8]. In our study, with a noninferiority margin set to 7%, 80% power, and 5% 1-sided type I error, based on a previously derived lower limit of the confidence interval [10], a total of 186 patients would have been required for each group to establish noninferiority. Considering a dropout rate of 10% would raise the required number of patients to 207 for each group and a total of 414 patients.
However, because of the low participation rate, only 83 patients were enrolled for the analysis. Nevertheless, we decided to proceed with the analysis because the efficiency of oral sulfate tablets for preoperative bowel preparation has not been evaluated or reported before. The post-hoc statistical power of the analysis was 0.1 alpha error and 13.1% power.
Categorical variables were analyzed using Pearson chi-square test or Fisher exact test as required. Continuous variables were analyzed using a t-test or a Wilcoxon rank-sum test following a test for normality; all statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R version 3.5.0 (The R Project, Vienna, Austria).
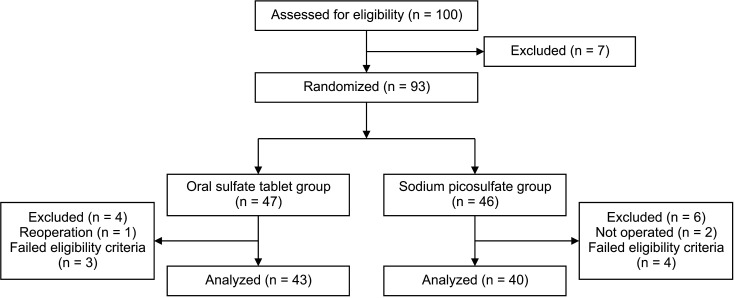
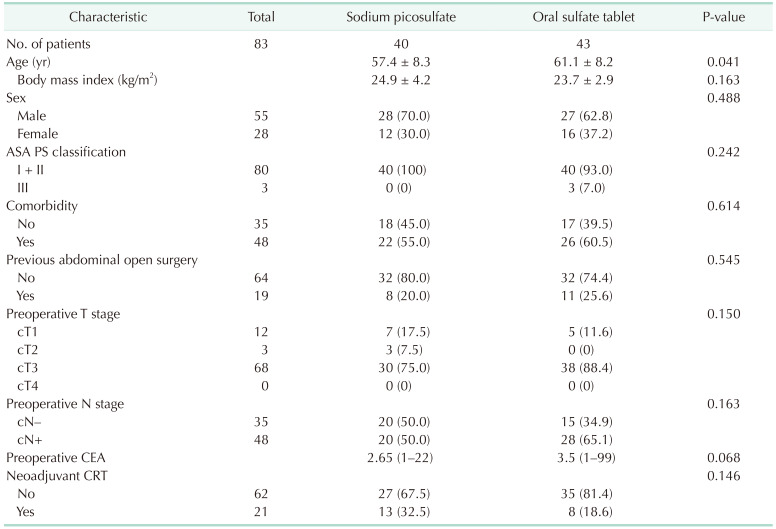
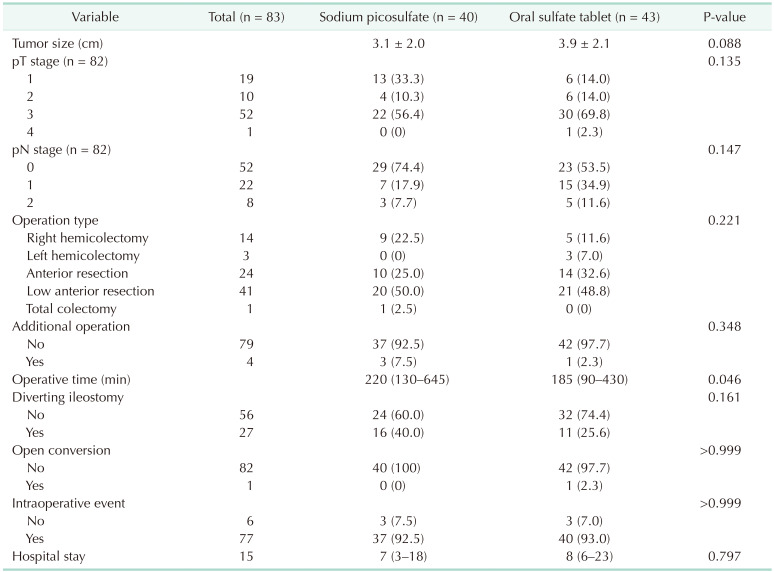
From October 2020 to December 2021, a total of 83 patients were included in the analysis: 40 patients in the sodium picosulfate group and 43 patients in the ORA·FANG group (Fig. 1). Table 1 shows the participants’ characteristics. The mean age of the patients was 57.4 ± 8.3 years in the sodium picosulfate group and 61.1 ± 8.2 years in the oral sulfate tablet group (P = 0.041). Body mass index, sex, American Society of Anesthesiologists physical status classification, comorbidity, previous abdominal surgery, preoperative stage, preoperative carcinoembryonic antigen, and neoadjuvant chemoradiotherapy were not significantly different between the 2 groups. The operation time was significantly different, with a median of 220 minutes (range, 130–645 minutes) required for preparation with sodium picosulfate and a median of 185 minutes (range, 90–430 minutes) required with oral sulfate tablets (P = 0.046) (Table 2). Tumor size, pathological stage, operation type, additional operation, diverting ileostomy, open conversion, intraoperative events, and hospital stay did not differ significantly between the 2 groups.
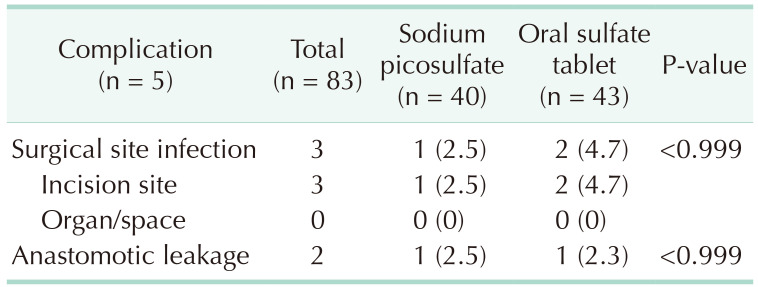
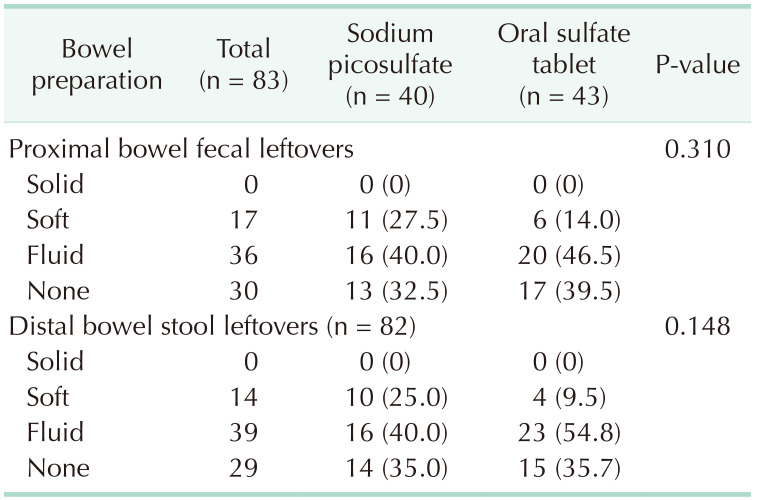
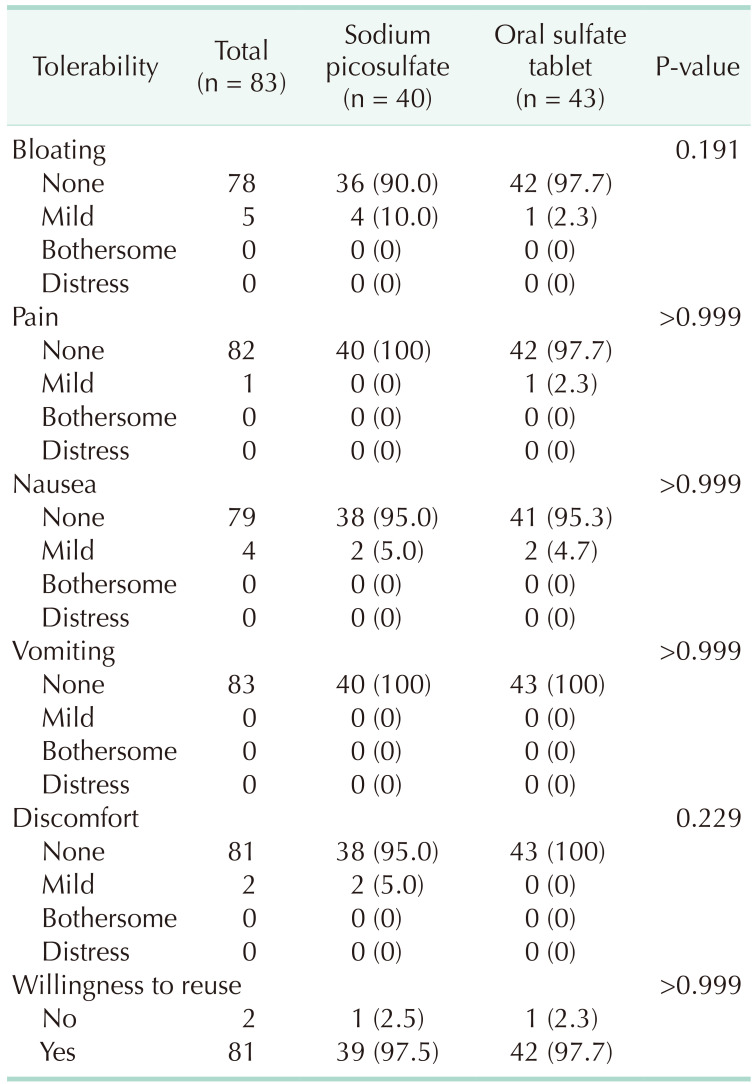
The primary outcome was a comparison of the surgical site infection rates between the 2 groups. Surgical site infection was found in 1 patient (2.5%) from the sodium picosulfate group and 2 patients (4.7%) from the oral sulfate tablet group, indicating no significant difference between the 2 groups (P > 0.999) (Table 3). The secondary outcomes studied were postoperative morbidity, the degree of bowel cleansing, and tolerability, none of which was found to differ significantly between the 2 groups. Specifically, postoperative morbidity was observed in 2 patients (5.0%) in the sodium picosulfate group and 3 patients (7.0%) in the oral sulfate tablet group (P > 0.999), whereas anastomotic leakage was present in 1 patient in each group. Regarding the degree of bowel cleansing in the proximal segment (Table 4), no significant difference was identified, with 11 patients (27.5%) in the sodium picosulfate group vs. 6 patients (14.0%) in the oral sulfate tablet group presenting soft to solid stools (P = 0.310). In the distal bowel segment, 10 patients (25.0%) bore soft to solid stools in the sodium picosulfate group, thus exhibiting no significant difference compared with the 4 (9.5%) patients identified in the oral sulfate tablet group (P = 0.310). There was no statistical significance between the 2 groups in the 4 tolerability parameters examined for each bowel preparation agent (Table 5), i.e., bloating (P = 0.191), pain (P > 0.999), nausea (P > 0.999), vomiting (P > 0.999), and discomfort (P = 0.229). The patients’ willingness to reuse the drugs did not differ significantly between the 2 groups (P > 0.999).
The effect of mechanical bowel preparation on surgical site infection has remained elusive [211]. In our study, we compared the surgical site infection rate, morbidity, degree of bowel cleansing, and tolerability between the oral sulfate tablet and sodium picosulfate groups. Bacteria may infect the abdominal wall or cavity upon fecal spillage during colorectal surgery; hence, many surgeons prefer a mechanical bowel preparation prior to surgery. In a survey of 74 Korean colorectal specialists, most surgeons (98.6%) required patients to undergo bowel preparation, and most patients (83.3%) used PEG [4]. PEG affords high bowel cleansing efficiency and safety but is accompanied by poor compliance owing to the large amounts that patients need to take and its unpleasant taste and flavor [1213]. Kim et al. [6] reported that sodium picosulfate showed higher bowel cleansing and lower adverse event rates than did the conventional 4-L PEG used for colonoscopy. Its tolerability was also better compared to that with PEG. Therefore, recently, sodium picosulfate has also been widely used in mechanical bowel preparation for colorectal surgery [814].
Recently, an oral sulfate agent in tablet form was introduced [7]. It contains 90% of the salts of a typical OSS formulation and simethicone as an antifoaming component. However, only 1 study on the efficiency and tolerability of oral sulfate tablets as a new bowel preparation agent has been reported. In a recent randomized trial, the efficiency, safety, and tolerability of oral sulfate tablets in bowel preparation for colonoscopy were compared with those of OSS. Bowel cleansing was achieved in 95.5% (107 of 112) of the patients in the oral sulfate tablet group, suggesting that oral sulfate tablets are non-inferior to OSS. Although their advantages in bowel preparation for colonoscopy were evident, there have been no studies on their efficiency and tolerability in bowel preparation for colorectal surgery. Therefore, we compared oral sulfate tablets with sodium picosulfate, the efficiency and tolerability of which have already been proven. The bowel clearance level is important for bowel preparation agents and may be the factor most related to surgical site infection; however, other factors, such as a change in the microbial environment in the bowel, edema, or dehydration of the intestine, are also likely to play a role in surgical site infection [15]. Therefore, this study focused on examining the surgical site infection rate associated with oral sulfate tablets rather than their bowel clearance level before surgery.
Since our study was conducted on participants who underwent minimally invasive surgery, we speculate that the number of incision site infections was lower than the expected 8% incidence, with no statistically significant difference between the 2 groups. This can be explained by the possible indirect relationship between surgical site infection rate and bowel cleansing. In both groups, there were no differences in clearance of the proximal (P = 0.310) and distal (P = 0.148) bowels. Further, bowel cleansing was adequate and mostly devoid of solid stool. This may explain the overall low rate of surgical site infection and the lack of differences between the 2 groups.
Although no significant difference was observed in tolerability between the 2 groups, patients in the oral sulfate tablet group exhibited no symptoms in more than 95% of the cases and for all parameters investigated. In addition, 97.7% of the patients were willing to reuse oral sulfate tablets. Similarly, in their study, Yang et al. [7] reported that 96.4% of the patients in the oral sulfate tablet group described the taste of the tablets to be good or tolerable, while only 79.6% of patients in the OSS group passed similar remarks. Similarly, the willingness to repeat the same preparation bore a significantly higher rate in the oral sulfate tablet group (76.8%) than in the OSS group (41.6%). Being tablets, oral sulfate tablets may be more convenient to take than solutions. They also contain fewer salts and require less water compared to OSS, resulting in superior tolerability and lower gastrointestinal discomfort. However, since OSS is not superior to PEG in terms of safety, oral sulfate tablets should be used with caution in patients with impaired renal function. Hence, oral sulfate tablets can be useful for patients with normal renal function who are reluctant to take bowel preparation agents as solutions.
The randomization and single-blinded design of our study are of particular importance. We randomly assigned our participants into 2 groups to minimize selection bias. Owing to the nature of the drugs and their administration, all participants knew which drug they had been administered, but the surgeons were unaware of which group each patient belonged to, both during surgery and while determining the degree of bowel cleansing intraoperatively. There have been very few studies on oral sulfate tablets; ours is the only one on preoperative bowel preparation using oral sulfate tablets. Our results suggest that oral sulfate tablets can be useful and are safe for preoperative bowel preparation.
Nevertheless, our study bears some limitations. First, to confirm noninferiority, 207 patients in each group and a total of 414 patients are required. However, patient enrollment was considerably slower than anticipated because we excluded patients with symptoms of obstruction or who had to undergo colonoscopy again for tattooing or polypectomy after bowel preparation while hospitalized for surgery. In addition, an attempt was made to recruit participants from multiple institutions; however, meeting the requirements for conducting a clinical trial, such as approval from IRBs, took a long time. Eventually, this study was conducted in a single institution. This slow enrollment made it difficult to extend the contract with the funding agency, leading to early termination of the study. Therefore, an adjustment of sample size was inevitable. Consequently, only 83 patients were examined, rendering our analysis insufficiently powerful to firm up our conclusions. Second, the surgical site infection rate as a primary endpoint was found to be particularly low, possibly reflecting the elective and only minimally invasive method of surgery implemented, as well as the exclusion from our study of patients with intestinal obstruction caused by tumors.
Although we could not establish the noninferiority of oral sulfate tablets to sodium picosulfate in terms of surgical site infection, we found no evidence suggesting that oral sulfate tablets are any less safe or tolerable than sodium picosulfate in preoperative bowel preparation.
Notes
References
1. Nichols RL, Condon RE, Gorbach SL, Nyhus LM. Efficacy of preoperative antimicrobial preparation of the bowel. Ann Surg. 1972; 176:227–232. PMID: 4562009.

2. Scarborough JE, Mantyh CR, Sun Z, Migaly J. Combined mechanical and oral antibiotic bowel preparation reduces incisional surgical site infection and anastomotic leak rates after elective colorectal resection: an analysis of colectomy-targeted ACS NSQIP. Ann Surg. 2015; 262:331–337. PMID: 26083870.

3. Kiran RP, Murray AC, Chiuzan C, Estrada D, Forde K. Combined preoperative mechanical bowel preparation with oral antibiotics significantly reduces surgical site infection, anastomotic leak, and ileus after colorectal surgery. Ann Surg. 2015; 262:416–425. PMID: 26258310.

4. Kang BM, Lee KY, Park SJ, Lee SH. Mechanical bowel preparation and prophylactic antibiotic administration in colorectal surgery: a survey of the current status in Korea. Ann Coloproctol. 2013; 29:160–166. PMID: 24032117.

5. Slim K, Vicaut E, Panis Y, Chipponi J. Meta-analysis of randomized clinical trials of colorectal surgery with or without mechanical bowel preparation. Br J Surg. 2004; 91:1125–1130. PMID: 15449262.

6. Kim YS, Hong CW, Kim BC, Han KS, Park JW, Choi HS, et al. Randomized clinical trial comparing reduced-volume oral picosulfate and a prepackaged low-residue diet with 4-liter PEG solution for bowel preparation. Dis Colon Rectum. 2014; 57:522–528. PMID: 24608310.

7. Yang HJ, Park DI, Park SK, Lee CK, Kim HJ, Oh SJ, et al. Novel sulfate tablet PBK-1701TC versus oral sulfate solution for colon cleansing: a randomized phase 3 trial. J Gastroenterol Hepatol. 2020; 35:29–36. PMID: 31396995.

8. Ikeda A, Konishi T, Ueno M, Fukunaga Y, Nagayama S, Fujimoto Y, et al. Randomized clinical trial of oral and intravenous versus intravenous antibiotic prophylaxis for laparoscopic colorectal resection. Br J Surg. 2016; 103:1608–1615. PMID: 27550722.

9. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999; 20:250–278. PMID: 10219875.
10. Tajima Y, Ishida H, Yamamoto A, Chika N, Onozawa H, Matsuzawa T, et al. Comparison of the risk of surgical site infection and feasibility of surgery between sennoside versus polyethylene glycol as a mechanical bowel preparation of elective colon cancer surgery: a randomized controlled trial. Surg Today. 2016; 46:735–740. PMID: 26319220.

11. Koskenvuo L, Lehtonen T, Koskensalo S, Rasilainen S, Klintrup K, Ehrlich A, et al. Mechanical and oral antibiotic bowel preparation versus no bowel preparation for elective colectomy (MOBILE): a multicentre, randomised, parallel, single-blinded trial. Lancet. 2019; 394:840–848. PMID: 31402112.

12. Golub RW, Kerner BA, Wise WE Jr, Meesig DM, Hartmann RF, Khanduja KS, et al. Colonoscopic bowel preparations: which one?: a blinded, prospective, randomized trial. Dis Colon Rectum. 1995; 38:594–599. PMID: 7774469.
13. Kastenberg D, Chasen R, Choudhary C, Riff D, Steinberg S, Weiss E, et al. Efficacy and safety of sodium phosphate tablets compared with PEG solution in colon cleansing: two identically designed, randomized, controlled, parallel group, multicenter phase III trials. Gastrointest Endosc. 2001; 54:705–713. PMID: 11726845.

14. Yoshioka K, Connolly AB, Ogunbiyi OA, Hasegawa H, Morton DG, Keighley MR. Randomized trial of oral sodium phosphate compared with oral sodium picosulphate (Picolax) for elective colorectal surgery and colonoscopy. Dig Surg. 2000; 17:66–70. PMID: 10720834.

15. An SH, Youn MK, Kim IY. Effect of laparoscopic surgery on the risk for surgical site infections in colorectal resection: results from the Health Insurance Review & Assessment Service Database. Ann Surg Treat Res. 2020; 98:315–323. PMID: 32528911.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download