Abstract
Purpose
Some studies have suggested that circumferential tumor location (CTL) of rectal cancer may affect oncological outcomes. However, studies after preoperative chemoradiotherapy (CRT) are rare. This study aimed to evaluate the impact of CTL on oncologic outcomes of patients with mid to low rectal cancer who received preoperative CRT.
Methods
Patients with mid to low rectal cancer who underwent total mesorectal excision after CRT from January 2013 to December 2018 were included in this retrospective study. The impact of CTL on the pathological circumferential resection margin (CRM) status, local recurrence-free survival (LRFS), disease-free survival (DFS), and overall survival (OS) was analyzed.
Results
Of the 381 patients, 98, 70, 127, and 86 patients were categorized into the anterior, posterior, lateral, and circumferential tumor groups, respectively. Tumor location was not significantly associated with the pathological CRM involvement (anterior, 12.2% vs. posterior, 14.3% vs. lateral, 11.0% vs. circumferential, 17.4%; P = 0.232). Univariate analyses revealed no correlation between CTL and 3-year LRFS (93.0% vs. 89.1% vs. 91.5% vs. 88%, P = 0.513), 3-year DFS (70.3% vs. 70.2% vs. 75.3% vs. 75.7%, P = 0.832), and 5-year OS (74.7% vs. 78.0% vs. 83.9% vs. 78.2%, P = 0.204). Multivariate analysis identified low rectal cancer and pathological CRM involvement as independent risk factors for all survival outcomes (all P < 0.05).
Go to : 
The rectum is located inside the pelvis and close to the adjacent organs; hence, it is important to perform appropriate radical resection of rectal cancer. Total mesorectal excision (TME) is a standard surgical treatment for rectal cancer [123], but the local recurrence rate after surgery is 10%–20% [45]. Preoperative chemoradiotherapy (CRT) has currently been recommended for patients with advanced rectal cancer to improve these outcomes [67].
The anterior mesorectum is thinner than the posterior mesorectum, and the anterior surface of the rectum is surrounded closely by structures such as the vagina, seminal vesical, and prostate [8910]. Therefore, because of these differences, it has been suggested that circumferential tumor location (CTL) may influence the risk of circumferential resection margin (CRM) involvement, which may lead to differences in oncological outcomes [1112131415]. Some studies have reported that anterior rectal cancer showed a significantly higher incidence of local recurrence and overall survival (OS) than non-anterior rectal cancer [11]; other reports have presented that the anterior location of rectal cancer was not associated with local recurrence and OS [1314]. Nonetheless, all of these studies included patients who did not undergo CRT before surgery. Therefore, concerning the standard treatment currently being implemented, whether CTL is a significant factor in the oncological outcome has not yet been determined.
Thus, this study aimed to evaluate the impact of CTL on the CRM and oncologic outcomes in mid to low rectal cancer patients who underwent TME following CRT.
Go to : 
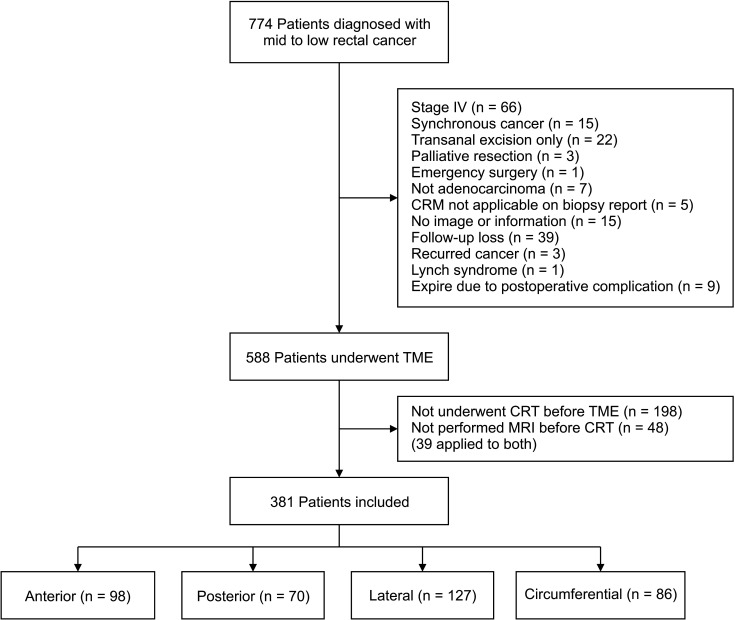
This study was approved by the Institutional Review Board of Chonnam National University Hwasun Hospital (No. CNUHH-2021-243). Due to the retrospective nature of our study, the requirement for informed consent was waived. We retrospectively reviewed prospectively collected data of 774 consecutive patients who underwent surgical treatment for mid to low rectal cancer (<10 cm from the anal verge) at our institution between January 2013 and December 2018 were selected. Patients with stage IV disease, synchronous or recurred cancer, Lynch syndrome, who underwent transanal excision, palliative resection, or emergency surgery and died within 30 days after surgery were excluded. Patients who did not receive neoadjuvant CRT or cases in which MRI was not performed before CRT were also excluded.
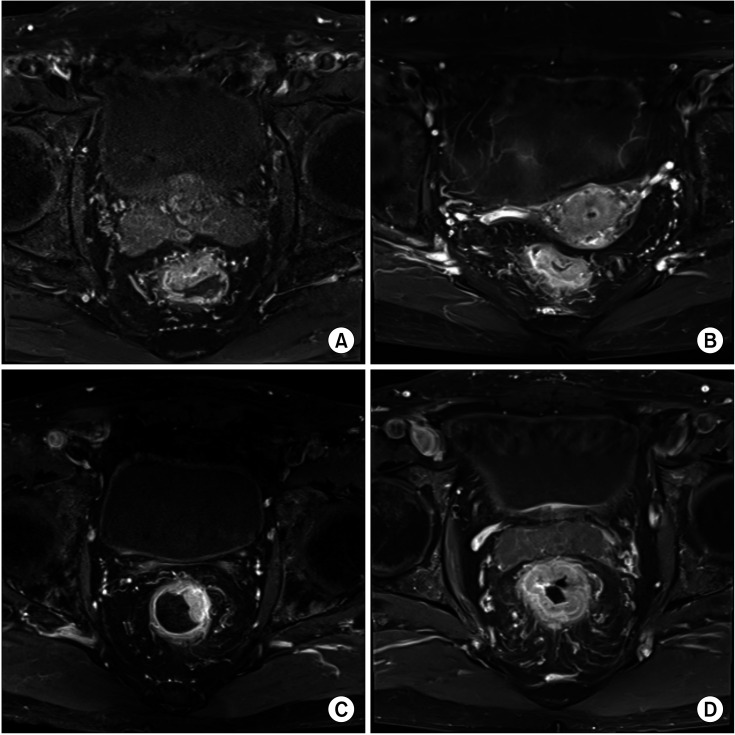
We retrospectively reviewed the data of MRI findings performed before CRT to identify CTL. We classified CTL into 4 groups: anterior, posterior, lateral, and circumferential. The first 3 locations were determined based on whether the location of the deepest tumor infiltration was within the 11–1 o’clock, 5–7 o’clock, and 2–4 or 8–10 o’clock intervals. Circumferential tumors were defined as those invading more than 75% of the rectal circumference (Fig. 1).
All included patients received 50.4 Gy of radiation throughout the pelvis with concurrent fluoropyrimidine-based chemotherapy [7]. Surgery was performed 6–10 weeks after CRT [7], and TME was performed routinely based on the principle of oncological radical resection [16].
The clinical and pathologic staging was determined using the classification system of the American Joint Committee on Cancer 8th edition [17]. Mid and low rectal cancers were defined as the tumors in which distal margins are located 5–10 cm and <5 cm above the anal verge, respectively. Tumor regression grading (TRG) was classified into the following scales [18]: grade 1, minor regression (obvious fibrosis in ≤25% of the tumor mass); grade 2, moderate regression (obvious fibrosis in 26%–50% of the tumor mass); grade 3, major regression (>50% tumor regression); and grade 4, complete regression (only fibrotic mass). Using the histopathological examination, tumors that infiltrated the CRM or were <1 mm from the CRM were defined as positive CRM [19].
Patients have been followed up every 6 months, and laboratory tests, chest CT, and abdominopelvic CT were conducted at each visit. Surveillance colonoscopy was performed at 1, 3, and 5 years after surgery. Recurrence was defined as the reappearance of the disease identified using clinical, radiologic, or pathologic examination. Local recurrence-free survival (LRFS) was measured as the time from surgery to the date of local recurrence or death. Disease-free survival (DFS) was defined as the period from surgery to the date of local recurrence, distant metastasis, or death. OS was calculated as the time between surgery and all-cause death.
The Student t-test or Mann-Whitney U test was performed to analyze continuous variables, and the chi-square test or Fisher exact test was performed for categorical variables. The Kaplan-Meier method and logistic regression analysis were performed to identify survival outcomes and risk factors for LRFS, DFS, and OS. Variables with a P-value of <0.05 in univariate analyses and CTL were included in multivariate analyses. Cox proportional hazard regression analysis was performed to determine the independent risk factors for survival outcomes with the calculation of hazard ratios (HRs) and 95% confidence intervals. A P-value of <0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics ver. 25.0 (IBM Corp., Armonk, NY, USA).
Go to : 
A total of 381 patients were included in our study (Fig. 2). Based on CTL, 98, 70, 127, and 86 patients had anterior, posterior, lateral, and circumferential tumors, respectively.
The clinicopathologic characteristics of the patients based on CTL are shown in Table 1. In the circumferential tumor group, the ratio of tumor size of >5 cm was significantly higher than that in the other groups (anterior, 7.3% vs. posterior, 11.6% vs. lateral, 13.5% vs. circumferential, 24.4%; P = 0.003). The clinical T stage (clinical T stage 3/4; 85.7% vs. 90.0% vs. 88.2% vs. 98.9%; P = 0.003), clinical N stage (clinical N stage 1/2; 38.8% vs. 48.6% vs. 41.7% vs. 61.6%; P = 0.001), and pathological T stage (pathological T stage 3/4; 54.1% vs. 52.9% vs. 48.0% vs. 74.4%; P = 0.003) differed significantly between the 4 groups. Regarding TRG, the circumferential tumor group had a lower rate of complete regression than the other groups (16.3% vs. 15.7% vs. 21.3% vs. 7.0%; P = 0.037). On the other hand, the pathological CRM status did not show a significant difference between the 4 groups (12.2% vs. 14.3% vs. 11.0% vs. 17.4%; P = 0.232). Other clinicopathological characteristics were similar in all groups (all P > 0.05).
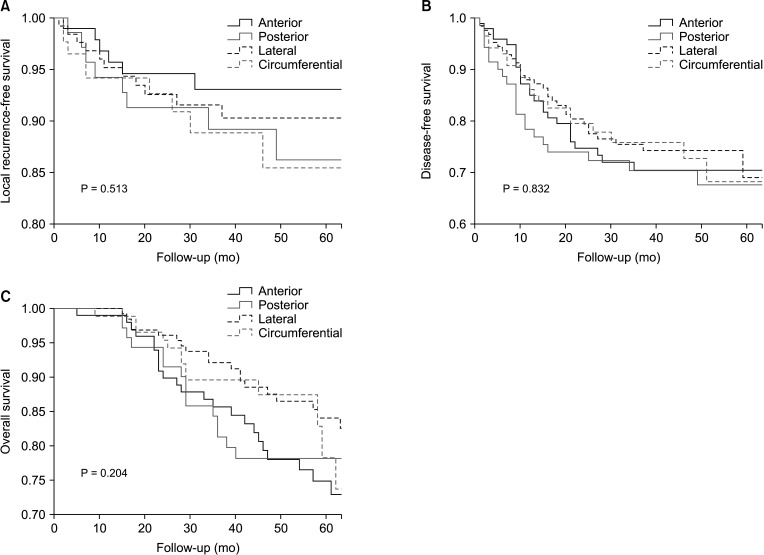
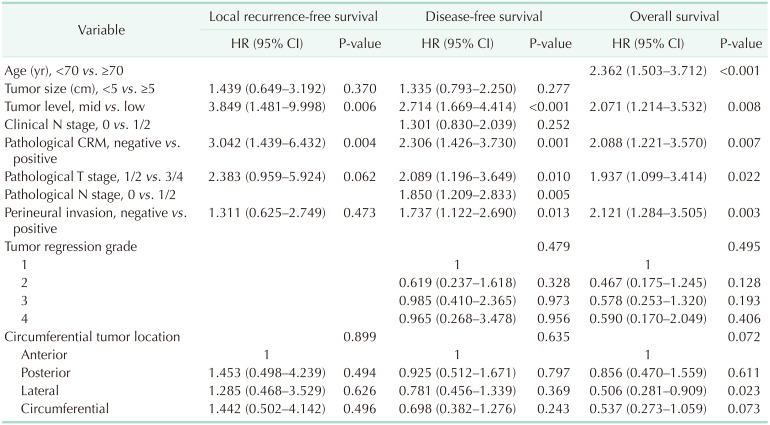
Univariate analyses were performed to identify the risk factors for survival outcomes in Table 2. Low rectal cancer, positive CRM, pathological T stage 3/4, and perineural invasion were significant risk factors in all survival outcomes (P < 0.05). Tumor size of >5 cm was associated with LRFS and DFS, and the pathological N stage 1/2, vascular invasion, and lymphatic invasion were relevant factors for DFS and OS. In addition, ≥70 years of age was relevant to OS and the clinical N stage 1/2 to DFS. CTL was not significantly associated with any survival outcome (LRFS, P = 0.513; DFS, P = 0.832; OS, P = 0.204) (Fig. 3).
The significant risk factors in univariate analyses and CTL were used as variables in multivariate analyses. Low rectal cancer (LRFS: HR = 3.809, P = 0.007; DFS: HR = 2.714, P < 0.001; OS: HR = 2.071, P = 0.008) and positive CRM (LRFS: HR = 2.859, P = 0.011; DFS: HR = 2.306, P = 0.001; OS: HR = 2.099, P = 0.007) were independent risk factors for all survival outcomes. The pathological T stage 3/4 (DFS: HR = 2.089, P = 0.010; OS: HR = 1.937, P = 0.022) and perineural invasion (DFS: HR = 1.737, P = 0.013; OS: HR = 2.121, P = 0.003) were significantly associated with DFS and OS. The pathological N stage 1/2 was an independent factor for DFS (HR = 1.850, P = 0.005), and ≥70 years of age significantly increased the risk of OS (HR = 2.362, P < 0.001). CTL was not an independent factor for survival outcomes (LRFS, P = 0.899; DFS, P = 0.635; OS, P = 0.072) (Table 3).
Go to : 
We analyzed the impacts of CTL and the significant risk factors for recurrence and survival in patients with mid and low rectal cancer who received TME following CRT. CTL was neither significantly associated with positive CRM nor a significant risk factor for recurrence and survival outcomes.
Several studies have shown conflicting results of oncological outcomes based on CTL in patients with rectal cancer. Some studies have presented a significant difference in local recurrence regarding CTL [1112]; however, other studies have shown that the anterior location of rectal cancer was not a significant factor in local recurrence [1314]. In addition, one study revealed significant differences in OS depending on CTL [11], but most other studies showed no significant differences in OS [131415]. These different oncologic outcomes may be attributed to differences in the proportions of patients who received preoperative CRT, group classification criteria, and inclusion of the circumferential tumor. In our study, preoperative CRT was performed in all patients, and the circumferential tumors were included. Therefore, tumor regression was followed by CRT, which should influence CTL in the CRT state. Subsequently, there was no significant difference in both local recurrence and OS based on CTL.
Because of the difference in thickness and area of the anterior and posterior mesorectum [9], it could be assumed that the proportion of positive CRM might be higher in the anterior group than in the posterior group, and the local recurrence rate might be high consequently. However, a significant difference in positive CRM depending on whether the tumor was located anteriorly or posteriorly and also in local recurrence and OS were not observed in this study. In multivariate analyses, peripheral infiltration, such as the pathological CRM involvement or perineural invasion, was a significant factor associated with oncologic outcomes. Moreover, low rectal cancer had a more significant impact on local recurrence and survival than mid rectal cancer. Despite structural differences based on the rectal tumor location, CRT might have reduced the impact on CTL; however, the impacts of tumor level and peripheral infiltration might still remain after CRT, leading to worse oncologic outcomes.
Since circumferential tumors have a larger tumor size and higher clinical or pathological T stage than other tumors, it was considered that TRG grade was affected showing a significant difference between tumor groups. As TRG has been suggested as a significant factor in local recurrence and systemic recurrence [20], it was assumed that the oncologic outcome in circumferential tumor might show a significant difference from other tumors. However, in our univariate analysis, TRG showed significant differences in either DFS or OS, but CTL was not a significant factor on oncologic outcomes.
The present study has several limitations. First, it was difficult to rule out selection bias because of its retrospective design. Second, the number of patients included in each group was small because the CTL was subdivided into 4 groups. Third, the location was classified using MRI before CRT; however, location classification might vary depending on the subjectivity of the reader. Tumor location classification was based on CTL recorded by a radiologist. However, in the absence of content corresponding to the radiology report, the location was determined through consultation. Fourth, the location of the deepest tumor infiltration identified using MRI, and that of the pathological CRM involvement, might not be corresponding. Biopsy reports documenting CTL identified for the CRM involvement, or prospective studies, would be required for accurately identifying the pathological CRM involvement site. However, although many studies have revealed the outcomes of rectal cancer based on CTL, the present study might be meaningful because studies in which all included patients who underwent CRT are rare. In addition, as circumferential tumor tended toward worse survival [13], it has been excluded from the inclusion criteria in several studies [1215]. To the best of our knowledge, studies analyzing local recurrence and survival outcomes, including circumferential tumor, in all subjects who underwent preoperative CRT, have not been published yet.
In conclusion, CTL of mid to low rectal cancer after CRT was not significantly associated with the pathological CRM status, local recurrence, and survival. Further large-scale prospective studies would be required to clearly confirm the impact of CTL in patients with rectal cancer treated by TME following CRT.
Go to : 
Notes
The abstract of this study was presented at the meeting of the Asia-Pacific Congress of Endoscopic and Laparoscopic Surgeons of Asia, Hong Kong, November 20 to 21, 2021.
Go to : 
References
1. Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg. 1998; 133:894–899. PMID: 9711965.
2. Kapiteijn E, Putter H, van de Velde CJ. Cooperative investigators of the Dutch ColoRectal Cancer Group. Impact of the introduction and training of total mesorectal excision on recurrence and survival in rectal cancer in The Netherlands. Br J Surg. 2002; 89:1142–1149. PMID: 12190680.

3. Wibe A, Møller B, Norstein J, Carlsen E, Wiig JN, Heald RJ, et al. A national strategic change in treatment policy for rectal cancer: implementation of total mesorectal excision as routine treatment in Norway: a national audit. Dis Colon Rectum. 2002; 45:857–866. PMID: 12130870.

4. Banwell VC, Phillips HA, Duff MJ, Speake D, McLean C, Williams LJ, et al. Five-year oncological outcomes after selective neoadjuvant radiotherapy for resectable rectal cancer. Acta Oncol. 2019; 58:1267–1272. PMID: 31237192.

5. Dassanayake BK, Samita S, Deen RY, Wickramasinghe NS, Hewavisenthi J, Deen KI. Local recurrence of rectal cancer in patients not receiving neoadjuvant therapy: the importance of resection margins. Ceylon Med J. 2011; 56:159–161. PMID: 22298209.

6. Augestad KM, Lindsetmo RO, Stulberg J, Reynolds H, Senagore A, Champagne B, et al. International preoperative rectal cancer management: staging, neoadjuvant treatment, and impact of multidisciplinary teams. World J Surg. 2010; 34:2689–2700. PMID: 20703471.

7. Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. NCCN guidelines insights: rectal cancer, version 6.2020. J Natl Compr Canc Netw. 2020; 18:806–815. PMID: 32634771.

8. Jhaveri KS, Hosseini-Nik H. MRI of rectal cancer: an overview and update on recent advances. AJR Am J Roentgenol. 2015; 205:W42–W55. PMID: 26102418.

9. Kang BM, Park YK, Park SJ, Lee KY, Kim CW, Lee SH. Does circumferential tumor location affect the circumferential resection margin status in mid and low rectal cancer? Asian J Surg. 2018; 41:257–263. PMID: 28118954.

10. Lindsey I, Guy RJ, Warren BF, Mortensen NJ. Anatomy of Denonvilliers’ fascia and pelvic nerves, impotence, and implications for the colorectal surgeon. Br J Surg. 2000; 87:1288–1299. PMID: 11044153.

11. Chan CL, Bokey EL, Chapuis PH, Renwick AA, Dent OF. Local recurrence after curative resection for rectal cancer is associated with anterior position of the tumour. Br J Surg. 2006; 93:105–112. PMID: 16302179.

12. Franz C, Lang HM, Ghamarnejad O, Khajeh E, Mehrabi A, Ulrich A, et al. Prognostic impact of ventral versus dorsal tumor location after total mesorectal excision of rectal cancer. Ann Surg Oncol. 2020; 27:430–438. PMID: 31549320.

13. García-Granero E, Faiz O, Flor-Lorente B, García-Botello S, Esclápez P, Cervantes A. Prognostic implications of circumferential location of distal rectal cancer. Colorectal Dis. 2011; 13:650–657. PMID: 20236143.

14. Lee SH, Hernandez de Anda E, Finne CO, Madoff RD, Garcia-Aguilar J. The effect of circumferential tumor location in clinical outcomes of rectal cancer patients treated with total mesorectal excision. Dis Colon Rectum. 2005; 48:2249–2257. PMID: 16400512.

15. Wang X, Chen G, Zhang Y, Ghareeb WM, Yu Q, Zhu H, et al. The impact of circumferential tumour location on the clinical outcome of rectal cancer patients managed with neoadjuvant chemoradiotherapy followed by total mesorectal excision. Eur J Surg Oncol. 2020; 46:1118–1123. PMID: 32113887.

16. MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993; 341:457–460. PMID: 8094488.

17. Weiser MR. AJCC 8th edition: colorectal cancer. Ann Surg Oncol. 2018; 25:1454–1455. PMID: 29616422.

18. Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005; 23:8688–8696. PMID: 16246976.

19. Mukkai Krishnamurty D, Wise PE. Importance of surgical margins in rectal cancer. J Surg Oncol. 2016; 113:323–332. PMID: 27094456.

20. Yoon WH, Kim HJ, Kim CH, Joo JK, Kim YJ, Kim HR. Oncologic impact of pathologic response on clinical outcome after preoperative chemoradiotherapy in locally advanced rectal cancer. Ann Surg Treat Res. 2015; 88:15–20. PMID: 25553320.

Go to : 




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download