INTRODUCTION
The current standard of treatment for locally advanced rectal cancer is neoadjuvant chemoradiotherapy (nCRT) followed by radical surgery and adjuvant chemotherapy [
1]. Despite the oncological benefit of this conventional treatment, it may cause several adverse effects, such as decreased quality of life, impaired bowel function, fecal incontinence, and genitourinary dysfunction [
2]. Thus, organ-preserving strategies, such as “watch and wait” and local excision, have been widely studied, especially in patients expected to have good oncologic outcomes [
34]. To provide patient-specific tailored treatment, it is important to predict the response to nCRT in the management of locally advanced rectal cancer.
Over the past few decades, there have been many studies on predictive biomarkers for response to nCRT in patients with rectal cancer [
5]. Many clinicopathological factors, such as TNM stage, tumor size, differentiation, location, budding, and pretreatment carcinoembryonic antigen, have been presented as predictors of response to nCRT [
56]. MRI and PET have also been used to assess the response to nCRT [
6]. Tumor environments, such as immune cell composition, cytokines, and chemokines, gut microbiome, as well as molecular markers, such as gene mutations, micro RNA, and circulating tumor DNA, have recently received a lot of attention [
56]. However, there are no reliable predictors for tumor response to nCRT in patients with locally advanced rectal cancer [
56].
Microsatellite instability (MSI) is a change in the length of tandemly repeated DNA sequences owing to the failure of the DNA mismatch repair (MMR) system [
7]. Currently, MSI testing is recommended for all patients with newly diagnosed rectal cancer [
1]. MSI testing is used not only to detect cases of hereditary nonpolyposis colorectal cancer but also as a prognostic marker and predictor of chemoresistance [
7]. However, there is limited evidence of an association between MSI and response to nCRT [
8].
Therefore, we designed this study to investigate the association between MSI and tumor response to nCRT in patients with rectal cancer.
Go to :

METHODS
We retrospectively reviewed the data of patients with rectal cancer from 2 tertiary hospitals (Chonnam National University Hwasun Hospital [CNUHH] and Seoul National Universiy Bundang Hospital [SNUBH]) and included patients who underwent nCRT, followed by radical surgery. The study period was from the time when the MSI test began as a routine exam (2007 in SNUBH and 2014 in CNUHH) to December 2020. Patients with recurrent rectal cancer or distant metastases were excluded from this study. After obtaining approval from the relevant Institutional Review Board of each institution (CNUHH, No. CNUHH-2022-107; SNUBH, No. B-2207-769-103), clinicopathological data, such as sex, age, body mass index, clinical and pathological stages, tumor differentiation, and microsatellite status, were collected. Informed consent was waived by the Institutional Review Board of each institution for its retrospective nature.
All patients underwent colonoscopy, abdominopelvic and chest CT, and rectal MRI for local staging. Clinical T and N stages were mainly determined using rectal MRI. The tumor location was classified according to the distance of the tumor from the anal verge assessed using rigid rectoscopy before nCRT; lower rectum (0–5 cm), mid-rectum (6–10 cm), and upper rectum (11–15 cm).
The basic principles of nCRT and radical surgery were similar in both institutions. Patients with clinical T3–4 or N1–2 rectal cancers or those with low rectal cancer of cT2N0 received 45.0–50.4 Gy radiation with 5-fluorouracil/leucovorin or capecitabine for sphincter-preserving surgery, followed by radical surgery 6–10 weeks after the completion of neoadjuvant therapy. All the patients underwent total mesorectal excision with regional lymphadenectomy. According to the National Comprehensive Cancer Network guidelines [
1], adjuvant chemotherapy is recommended for all patients irrespective of the pathologic stage, which is determined by the attending physician based on the general condition of the patient. The final pathological features were restaged according to the 8th edition of the American Joint Committee on Cancer TNM staging system.
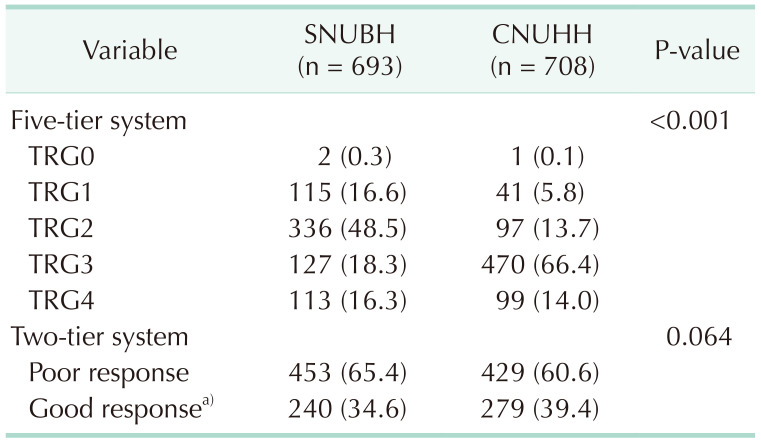
The tumor regression grade (TRG) was classified according to the Dworak or Rödel grading system [
910]. Because almost all patients with pathologic complete response (CR) had no MSI testing results owing to the absence of tumor tissue, we planned to explore the relationship between MSI and good response to nCRT. To compensate for the large difference in the degree of TRG evaluation between the 2 institutions (
Table 1), we had to define a good response to nCRT differently for each institution, as TRG3 or TRG4 by the Dworak grading system in SNUBH [
9] and tumor regression of ≥90% with fibrosis outgrowing the tumor mass by the Rödel grading system in CNUHH [
1011]. In this way, the proportion of good responses to nCRT was similar between the 2 institutions (34.6% in SNUBH and 39.4% in CNUHH, P = 0.064) (
Table 1).
Table 1
Tumor regression grade (TRG) according to the institution

MSI testing was performed using surgical specimens. As previously described, PCR analyses were performed using DNA extracted from paraffin-embedded tumors and normal tissues from each patient [
12]. Five microsatellite markers (BAT-25, BAT-26, D2S123, D5S346, and D17S250), recommended by the National Cancer Institute workshop on MSI, were used to determine the MSI status [
13]. The PCR products from tumor DNA were compared with those of DNA from normal mucosa, and tumors with ≥2 microsatellite markers displaying shifted alleles were classified as MSI-high (MSI-H). Samples with only 1 marker with a shifted allele were classified as MSI-low (MSI-L), and those with all markers displaying identical patterns in the tumor and normal tissues were classified as microsatellite stable (MSS).
Categorical variables were compared using the chi-square or Fisher exact test, and continuous variables were compared using the Student t-test. Multivariable logistic regression was performed using the backward stepwise method with variables with a P-value of <0.05 and microsatellite status. All results were considered to be statistically significant at P < 0.05. Statistical analyses were performed using IBM SPSS Statistics ver. 27.0 (IBM Corp., Armonk, NY, USA).
Go to :

RESULTS
A total of 1,401 patients were included (693 in SNUBH and 708 in CNUHH), and 910 of them (65.0%) had MSI results. Among the patients with MSI results, 868 (95.4%) showed MSS, 28 (3.1%) showed MSI-L, and only 14 (1.5%) showed MSI-H (
Fig. 1).
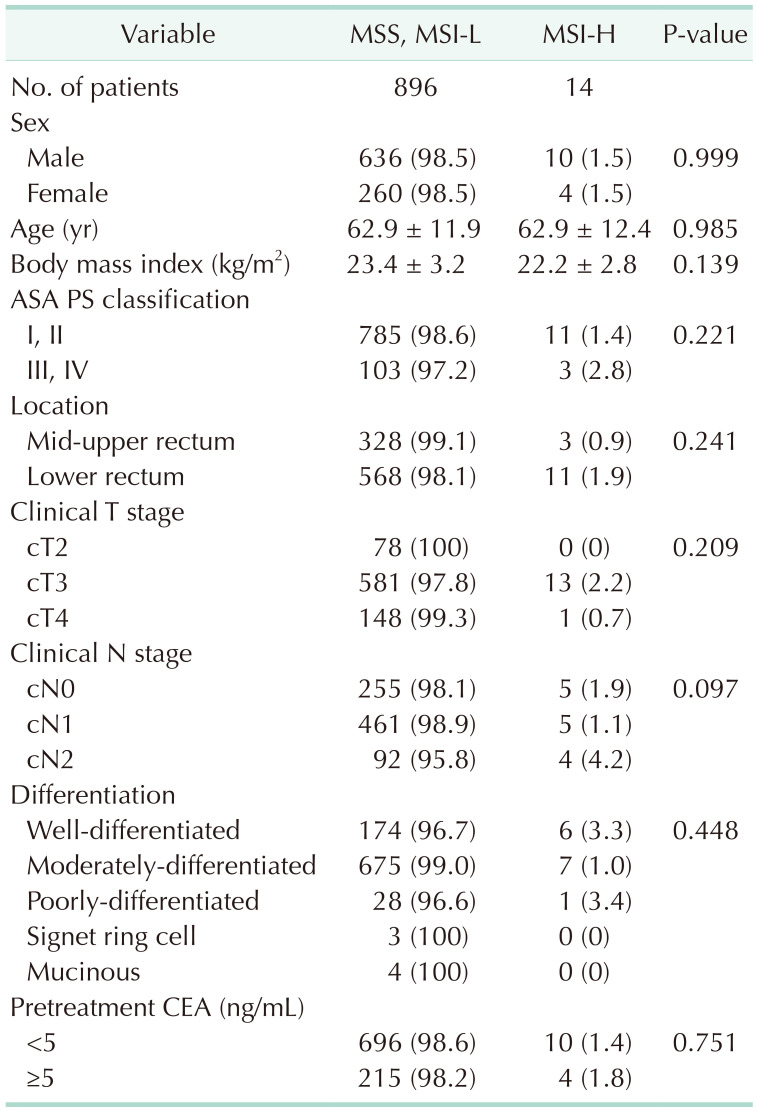
Table 2 shows the correlation between MSI status and clinical variables, and no variables were significantly associated with MSI-H.
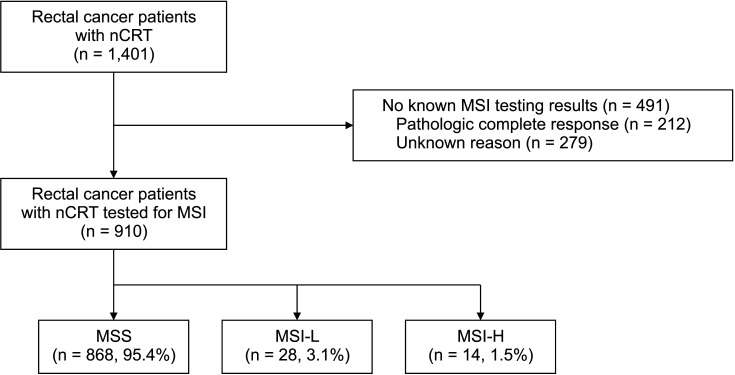 | Fig. 1A study flowchart. nCRT, neoadjuvant chemoradiotherapy; MSI, microsatellite instability; MSS, microsatellite stable; MSI-L, MSI-low; MSI-H, MSI-high.
|
Table 2
Correlation between microsatellite status and clinical variables

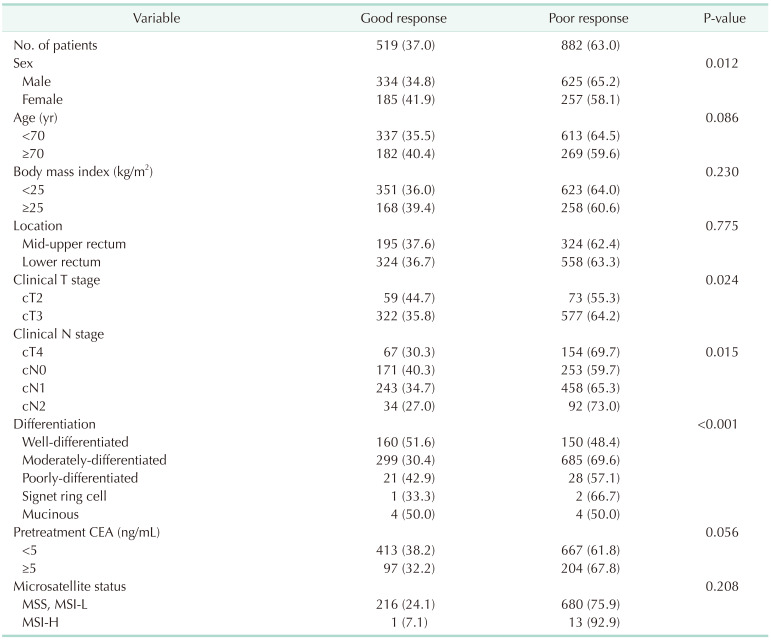
Among the included patients, 15.1% (212 of 1,401) showed a CR to the primary tumor (TRG4). TRG3 tumors comprised 18.3% (127 of 693) of SNUBH patients and 66.4% (470 of 708) of CNUHH patients. Evaluation of the TRG showed a large difference between the 2 institutions (P < 0.001) (
Table 1). According to the two-tier system, patients with a good response to nCRT (TRG3 and TRG4 in SNUBH, tumor regression ≥90% with fibrosis in CNUHH) comprised 37.0% (519 of 1,401), which was similar between the 2 institutions (34.6%
vs. 39.4%, P = 0.064).
Table 3 shows the univariate analysis of the predictive factors for a good response to nCRT. Female sex (41.9%
vs. 34.8%, P = 0.012), clinical T stage (cT2
vs. cT3, cT4; 44.7%
vs. 35.8%, 30.3%; P = 0.024), clinical N stage (cN0
vs. cN1, cN2; 40.3%
vs. 34.7%, 27.0%; P = 0.015), and tumor differentiation (well-differentiated
vs. moderately-differentiated, poorly-differentiated, signet ring cell, mucinous: 51.6%
vs. 30.4%, 42.9%, 3.3%, 50.0%; P < 0.001) were significantly associated with good response to nCRT. MSI-H had a tendency toward a low rate of good response to nCRT, which was not statistically significant (7.1%
vs. 24.1%, P = 0.208).
Table 3
Univariate analysis of predictive factors for a good response to neoadjuvant chemoradiation

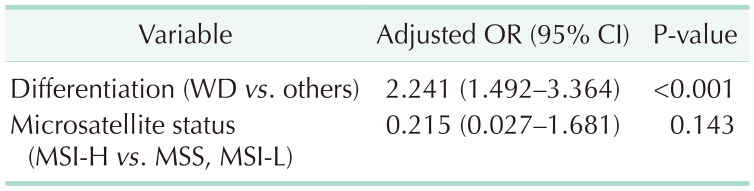
Multivariate logistic regression analysis with significant variables in univariate analysis (sex, clinical T and N stages, and tumor differentiation) and microsatellite status showed that well-differentiated tumors were the only independent predictive factor for good response to nCRT (adjusted odds ratio [OR], 2.241; 95% confidence interval [CI], 1.492–3.364; P < 0.001) (
Table 4). Although microsatellite status remained a variable until the final step, it did not show statistical significance in predicting response to nCRT (adjusted OR, 0.215; 95% CI, 0.027–1.681; P = 0.143).
Table 4
Multivariate analysis of predictive factors for a good response to neoadjuvant chemoradiation

Go to :

DISCUSSION
In the present multicenter study in Korea, the proportion of MSI-H patients with rectal cancer was very low (1.5%). Tumor differentiation was the only independent predictive factor for a good response to nCRT, and MSI-H showed a tendency to predict a poor response to nCRT, which was not statistically significant.
Previous studies on the association between MSI-H or defective MMR (dMMR) and tumor response to nCRT in rectal cancer have presented conflicting results [
1415161718]. Some studies reported that MSI-H or dMMR were associated with a good response to nCRT [
1516]. Meillan et al. [
15] showed that dMMR was associated with tumor downstaging (69.6%
vs. 48.7%, P = 0.02). Other authors have reported that patients with dMMR had a pathologic CR rate of 27.6%, but patients with proficient MMR were not presented as controls [
16]. In contrast, a recent nationwide cohort study reported that MSI was associated with a reduction in pathological CR after nCRT for rectal cancer [
18]. Some other studies reported no association between MSI or MMR gene expression and tumor response to nCRT in rectal cancer [
1417]. In fact, the heterogeneity of previous studies, such as MSI and dMMR testing methods (immunohistochemistry [
151617] or PCR [
141516]), the definition of MSI (MSI-H [
1415] or MSI-H/MSI-L [
18]), and outcome variables (pathologic CR [
161718] or downstaging of tumors [
1415]), makes it more difficult to draw a definite conclusion. With these limitations, a recent meta-analysis reported no significant association between MSI and pathologic CR rate after nCRT in patients with rectal cancer [
8].
The present study showed a large difference in the proportion of patients with a good response to nCRT between the MSI-H and MSS/MSI-L groups (7.1% vs. 24.1%), suggesting that MSI-H may be associated with a poor response to nCRT. However, we did not find any statistically significant difference. The main reason for the lack of statistical significance was the small number of patients with MSI-H (n = 14). Despite collecting data from the 2 institutions, the proportion of MSI-H was unexpectedly low (1.5%) to collect enough data from patients to prove statistical significance. Nevertheless, the results of the present study provide insights into the relationship between MSI-H and the response to chemoradiotherapy.
The mechanism underlying the possible relationship between MSI-H and poor response after nCRT in rectal cancer remains unclear. Regarding chemotherapy, a defective DNA repair system may hinder 5-fluorouracil-induced apoptosis and cell cycle arrest, resulting in chemoresistance [
8]. However, this cannot translate to resistance to chemoradiotherapy. One possible explanation for this finding is the association with different tumor biology. MSI-H tumors are classified as consensus molecular subtype 1 and have a higher incidence of
BRAF mutations [
19], which have been reported to be associated with resistance to nCRT [
20]. In fact, some studies have reported that patients with MSI-H rectal cancers showed worse survival [
2122], which is in accordance with the poor response to nCRT in the present study. These results may be the basis for performing tailored treatments such as neoadjuvant immunotherapy in patients with MSI-H rectal cancer [
23] according to the results of pretreatment MSI testing on colonoscopic biopsy [
1822].
Interestingly, MSI-H tumors accounted for only 1.5% of the rectal cancers in the present study. In fact, colon and rectal cancers have different pathological and molecular characteristics, and rectal cancer has a lower incidence of MSI-H than colon cancer [
8]. Even considering this, the proportion of MSI-H in the present study is much lower than that in previous studies from Western countries (2.7%–7.9%) [
141518]. Our previous study on the prognosis of MSI-L in colon cancer similarly reported lower incidences of MSI-H (6.6%) and MSI-L (6.6%) in colon cancer than those in Western countries (15%–25%) [
7]. These observations strongly suggest ethnic differences in carcinogenesis and biological characteristics of patients with colorectal cancer in Korea, which means that optimized treatment for colorectal cancer is needed for Eastern patients.
In the present study, only 65% of the included patients showed MSI results. One reason is that many patients did not undergo MSI testing at the beginning of routine MSI testing at each institution. This may have acted as a selection bias and affected the final results of the analysis. The second and more important reason is that, in cases of pathologic CR, it is impossible to perform MSI testing with surgical tissue because tumor tissue does not remain. Therefore, almost all patients with TRG4 (15.1%, 212 of 1,401) did not have MSI results. As the results of the present study imply a poor response to nCRT in patients with MSI-H, pretreatment MSI testing using colonoscopic biopsy is needed to predict tumor response to nCRT, and further research is needed.
There are many TRG systems for gastrointestinal cancer after neoadjuvant therapy [
24]. Although both institutions used 5-tier TRG systems, the proportion of grades evaluated by each institution showed a large difference between the 2 institutions (
Table 1). Assuming that the tumor response will be similar when administered with the same chemoradiotherapy protocol, the difference in TRG between the 2 institutions may be due to the difference in TRG evaluation and not the difference in tumor response itself. Therefore, we had to adjust the proportion of good responses between the 2 institutions to be similar by using different definitions of good responses for each institution. Although the difference between the 2 institutions was adjusted for, a somewhat arbitrary two-tier system could be a limitation of this study.
The present study had several limitations. First, the number of MSI-H patients was so small that we failed to prove statistical significance despite showing a tendency for correlation between MSI-H and poor response to nCRT. In addition, we could not compare the survival outcomes according to MSI because of the low incidence of MSI-H. Second, patients with pathologic CR were excluded from the analysis because they had no MSI results, which may have acted as selection bias. Third, postoperative MSI results that can be influenced and changed by nCRT itself were utilized for analysis. Therefore, a prospective study using MSI results performed with preoperative colonoscopic biopsy is needed. Also, because of the retrospective study design, we could not include other molecular markers such as KRAS and BRAF in the analysis. Lastly, as stated above, the evaluation of the TRG was largely different between the 2 institutions. Nevertheless, the present study is significant in that we showed a very low incidence of MSI-H in Korean rectal cancer patients and the possibility of an association between MSI-H and a poor response to nCRT, suggesting a direction for future research.
In conclusion, MSI status was not associated with response to nCRT in patients with rectal cancer. Although MSI-H showed a tendency to predict a poor response to nCRT, we failed to prove statistical significance. Because the proportion of MSI-H in rectal cancer patients is very low, a large-scale multicenter study is needed to prove the possible association between MSI-H and poor response to nCRT in rectal cancer.
Go to :







 PDF
PDF Citation
Citation Print
Print






 XML Download
XML Download