Abstract
Purpose
We aimed to investigate the protective effect of sigma 1 receptor agonist and antagonist, PRE084 and BD1047, respectively, on 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis in mice.
Methods
Thirty male ICR mice were randomly divided into 5 groups: control, 50% ethanol, colitis, PRE084 + colitis, and combined (PRE084 + BD1047 + colitis). Colitis was induced by intrarectal administration of TNBS. PRE084 and BD1047 were injected daily, starting 3 days before colitis induction. Distal colon tissue was excised for histopathological evaluation, and levels of glutathione (GSH), superoxide dismutase (SOD), myeloperoxidase (MPO), and lipid peroxidation were determined.
Results
Colitis caused weight loss, mucosal damage, upregulation of tumor necrosis factor-α, interleukin (IL)-1β, IL-6, MPO, and thiobarbituric acid reactive substance activities, and downregulation of GSH and SOD activities. These changes caused by TNBS-induced colitis were significantly ameliorated by PRE084 pretreatment. However, the combined pretreatment with BD1047 significantly attenuated the protective effect of PRE084, thereby reverting to the colitis-induced state.
Conclusion
We conclude that the sigma 1 receptor agonist PRE084 exhibits significant protective effects against TNBS-induced colitis, which appears to be at least partly mediated by the inhibition of inflammation and oxidative stress, and enhancement of antioxidant properties. Collectively, these results suggest that PRE084 might be an effective drug for the treatment of ulcerative colitis.
Inflammatory bowel disease (IBD) is one of the most common inflammatory digestive diseases and is prevalent worldwide. It is caused by the interaction of genetic and environmental factors affecting the immune system, which in turn affects the gastrointestinal tract. However, the pathogenesis of this disease remains poorly understood [1]. IBD is a chronic disease characterized by inflammation of the intestine and relapsing and remitting of mucosal damage. Common symptoms include diarrhea, nausea, abdominal pain, fatigue, rectal bleeding, and weight loss [2]. IBD is primarily categorized into Crohn disease and ulcerative colitis (UC). Although Crohn disease and UC have similar symptoms, some differences exist. Both diseases can occur in adolescents and adults and are almost equally prevalent in men and women [3]. Currently, IBD is one of the most prevalent digestive diseases in developed countries such as the United States and Western European countries. In contrast, its prevalence is lower in South America and Eastern Europe [3]. However, owing to the adoption of the Western diet and lifestyle, the incidence and prevalence of IBD are increasing worldwide, including in Korea. During the 8 years between 2009 and 2016, the prevalence of IBD in Korea has increased by about 1.87 times [4].
UC lesions are characterized by localized inflammation in the mucosal and submucosal layers, as well as chronic intestinal inflammation that starts in the rectum and continually spreads to the rest of the colon, frequently affecting the appendix [5]. The characteristic clinical symptoms of UC include abdominal pain, rectal bleeding, and diarrhea, which can progress from mild to severe. Persistent UC eventually leads to the development of colorectal cancer. Currently, the pathogenesis of UC is poorly understood. Several categories of therapeutics, such as anti-inflammatory drugs and immunosuppressants, have improved life expectancy in patients with UC; however, effective therapies are still limited, and disease relapse is common [67]. Therefore, developing an effective therapeutic agent to manage UC would have clinical significance. Understanding UC pathogenesis can provide vital information for the development of new therapies. Many experimental models have been established to study UC. Among them, 2,4,6-trinitrobenzene sulfonic acid (TNBS)–induced colitis mimics many of the cellular and humoral immune characteristics of human UC [8]. This model has been proven valuable in studying and evaluating the effectiveness of therapeutics and is, therefore, widely used [9].
Multiple factors, including cytokines, reactive oxygen species (ROS) overproduction, and massive neutrophil infiltration, can cause UC by damaging the intestinal mucosa [10111213]. Mucosal inflammation and ulceration in UC are caused by activated cells of the immune system. T lymphocytes of the intestinal-associated immune system produce large amounts of cytokines, leading to mucosal damage in patients with UC. Cytokines and inflammatory mediators that play significant roles in the development and persistence of mucosal inflammation include tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, IL-12, and IL-23 [11]. Therefore, the study of cytokines is vital in understanding UC pathogenesis.
Several studies conducted to explore UC pathogenesis have suggested that the potential pathogenic mechanisms include the generation of oxygen-derived free radicals such as superoxide anions, lipid peroxides, and neutrophil infiltration [121314]. Hydrogen peroxide (H2O2) is produced by every cell in the body and plays important physiological roles in cellular processes but can cause disease when produced in excess [14]. Lipid peroxidation is known to be involved in the pathogenesis of UC [15]. Antioxidant agents such as vitamin E, catechin, and astaxanthin, are also known to inhibit lipid peroxidation products and consequently reduce mucosal inflammation in patients with IBD [1617]. The protective effect of these antioxidant enzymes strongly indicates the involvement of ROS in UC development. Neutrophil infiltration is positively correlated with UC development; this infiltration into intestinal tissue can be monitored by measuring myeloperoxidase (MPO) activity. Neutrophils activate immune cells primarily by generating chemokines and cytokines. They migrate to the inflammation site and reduce inflammation upon stimulation; however, continuous activation and overproduction of neutrophils cause inflammatory diseases, including UC [13].
Sigma receptors are classified into 2 types, namely sigma-1 and sigma-2. The activation of the sigma-1 receptor in neurodegenerative diseases is known to have a neuroprotective effect; however, the role of the sigma-2 receptor remains unknown [18]. Sigma-1 receptor is a transmembrane protein that functions as a chaperone and a pathological mediator. When activated by ligand binding, it increases the antioxidant activity in cells and relieves inflammation by acting on intracellular ion channels and activating protein kinases. The sigma-1 receptor also has neuroprotective effects [1920]. Recently, the sigma-1 receptor has been used to treat pain caused by enteritis and reportedly exerts anti-inflammatory effects through antioxidant action in UC [1921]. We presumed that the protective effect of sigma-1 receptor agonist PRE084 in UC might be because of the activation of antioxidant signaling pathways. However, little is known about the action mechanism of the sigma-1 receptor. Thus, we examined the protective effect(s) of the sigma-1 receptor on TNBS-induced colitis in mice and elucidated its anti-inflammatory action mechanism.
The experimental colitis protocol (No. CKU-2020-3) was approved by the Institutional Animal Care and Use Committee of Catholic Kwandong University. All the experimental protocols were performed in accordance with the guidelines of care and use of laboratory animals prepared by the National Institute of Health.
A total of 30 male ICR mice weighing 25–30 g (supplied by SamtakoBio, Osan, Korea) were used in the experiments. The animals were acclimatized for 14 days under a controlled temperature of 22℃ ± 2℃, a 12-hour light/dark cycle (lights on from 8:00 to 20:00 hours), and free access to food and water. The mice were randomly divided into 5 groups (6 mice/group): (1) control group (normal mice treated with normal saline); (2) ethanol group (normal mice treated with 50% ethanol); (3) TNBS-induced colitis group; (4) TNBS + PRE084 group; and (5) TNBS + PRE084 + BD1047 group.
Colitis was induced by intrarectal administration of 100 µL of 50-mg/mL TNBS in 50% ethanol solution, as described by Kiesler et al. [9]. This dose of TNBS was found to reproducibly induce colitis without mortality in the pilot experiments. Before the intrarectal injection of 50% ethanol or TNBS solution, the mice were fasted for 24 hours and lightly anesthetized with sodium pentobarbital (50 mg/kg) via intraperitoneal injection. A plastic, flexible catheter equipped with a 1-mL syringe was lubricated and inserted 4 cm into the rectum. In order to prevent the leakage of TNBS solution, the mice were held in a head-down position for 30 minutes after the injection. The body weight and health status of mice were monitored daily. Starting from 3 days prior to the injection of TNBS, PRE084 (sigma-1 receptor agonist, 0.5 mg/kg, dissolved in 2% dimethyl sulfoxide) or PRE084 (0.5 mg/kg) + BD1047 (sigma-1 receptor antagonist, 0.1 mg/kg) were injected intraperitoneally every day. No death was observed during the model development. All mice were sacrificed 3 days after intrarectal administration of TNBS. At the end of the experiment, all mice were fasted for 24 hours, weighed, and sacrificed. The distal colon was removed, gently opened along the antimesenteric border, rinsed in phosphate buffer solution (PBS), weighed, and divided into 3 sections. One section was immediately fixed in 10% paraformaldehyde solution for histological analysis. The other 2 sections were frozen in liquid nitrogen and stored at -80℃ to assess the cytokine levels, MPO activity, and thiobarbituric acid reactive substance (TBARS) assay.
Body weight was measured on days 0 and 3. Macroscopic scoring of colonic ulceration and inflammation was performed according to the protocol described by Min et al. [22]. The scores were as follows: 0 = no ulcer and no inflammation; 1 = no ulceration and local hyperemia; 2 = ulceration without hyperemia; 3 = ulceration and inflammation at only 1 site; 4 = 2 or more sites of ulceration and inflammation; 5 = ulceration extending more than 2 cm. Three independent experiments were performed to obtain the assessment score, and the average of the 3 scores was used as the macroscopic score.
After macroscopic analysis, a small portion of each colon was fixed in 10% formalin solution for 24 hours. Colon tissue sections were dehydrated with graded ethanol concentrations, passed through xylene, and embedded in paraffin. The paraffin sections (5 mm thick) were stained with H&E, and the severity of colitis was assessed.
The levels of TNF-α, IL-1β, and IL-6 in the colon supernatant were determined using mouse cytokine-specific ELISA kits, according to the respective manufacturer’s instructions. For this purpose, colon tissue samples were homogenized in 0.3-mL PBS/10-mg tissue at pH 7.2 and centrifuged at 3,000 ×g for 20 minutes at 4℃. Aliquots (100 µL) of the supernatants were then used for the assays. All samples were analyzed in triplicate and normalized to the total protein content of the sample. Tissue TNF-α and IL-1β levels were expressed in pg/mL.
Glutathione (GSH), superoxide dismutase (SOD), and MPO activities in the colon supernatant were determined using the respective ELISA kits, according to the manufacturer’s instructions. Briefly, colon tissues were homogenized and centrifuged at 18,000 ×g for 20 minutes at 4℃ to obtain the supernatant. GSH activity was measured using a 0.01 mol/L solution of 5,5’-dithiobis (2-nitrobenzoic acid) in methanol and Tris buffer (0.2 mol/L, pH = 8.2). The absorbance of the supernatant was measured at 412 nm, and GSH activity was expressed as U/mg protein in the wet tissues.
SOD activity in the colonic tissue supernatants was assessed using a SOD assay kit. This protocol uses xanthine and xanthine oxidase to generate superoxide radicals, which can react with p-iodonitrotetrazolium violet to form a red formazan dye. The dye intensity can be measured spectrophotometrically at 505 nm. The assay medium contained 0.01-M phosphate buffer (pH 6.8), 3-cyclohexylamino-1-propanesulfonic acid (CAPS) buffer solution (pH 10.2, 50-mM CAPS, 0.94-mM EDTA, saturated NaOH), substrate solution (0.05-mM xanthine, 0.025-mM iodonitrotetrazolium chloride), and 80-µL xanthine oxidase. SOD activity was expressed as U/mg protein in the wet tissues.
MPO activity served as a marker of neutrophil infiltration into the inflamed colonic mucosa. Colon tissues were homogenized in a potassium phosphate buffer (pH 6.0) containing 0.3% hexadecyl trimethyl ammonium bromide to inhibit the pseudoperoxidase activity of hemoglobin, as well as to solubilize membrane-bound MPO. The homogenate was sonicated for 10 seconds in 3 freeze-thaw cycles on ice. The homogenate was then centrifuged to obtain supernatant (18,000 ×g for 20 minutes at 4℃). The supernatant was used to determine MPO activity using an ELISA kit. Potassium phosphate buffer (pH 6.0, 618 µL) containing 0.5-mM O-dianisidine dihydrochloride and 0.05% hydrogen peroxide was added to 125 µL of the supernatant. Absorbance at 450 nm was measured at room temperature (25℃). MPO from human leukocytes was used as a standard for the assay. MPO activity was expressed as U/mg protein in the wet tissues.
Colon tissues were homogenized in 5 volumes of ice-cold 1.15% sodium chloride. The colon homogenate was centrifuged at 3,000 ×g for 10 minutes at 4℃. A 945-µL volume of the resulting supernatant was added to 5 µL of the test compound, followed by the addition of 50-µL FeSO4. The mixture was then incubated at 37℃ for 1 hour. The reaction was stopped by adding 200 µL of 35% perchloric acid (HClO4), and the mixture was then centrifuged at 3,000 ×g for 10 minutes. A 500-µL aliquot of the supernatant was heated with 500-µL TBARS, 750-µL PBS, and 100 µL sodium dodecyl sulfate for 60 minutes at 100℃. After cooling, an equivalent amount of n-butanol was added, and the tube was shaken vigorously for 1 minute and then centrifuged for 10 minutes at 4,000 ×g. The absorbance was measured spectrophotometrically at 532 nm. The tissue levels of TBARS were calculated from a standard curve plotted using different concentrations of 1,1,3,3-tetraethoxypropane and expressed as micromoles per gram of tissue (µM/g protein).
All the chemicals and drugs were of the highest purity and were commercially available. TNBS was purchased from Tokyo Kasei (Tokyo, Japan). PRE084 and (N-[2-(3,4-dichlorophenyl) ethyl]-N-methyl-2-(dimethylamino) ethylamine) (BD1047) were purchased from Tocris Bioscience (Minneapolis, MN, USA). TNF-α, IL-1β, and IL-6 cytokine ELISA kits were purchased from Invitrogen (Waltham, MA, USA). GSH peroxidase, and SOD, MPO, and TBARS ELISA kits were purchased from Cayman Chemical (Ann Arbor, MI, USA). PRE084 was dissolved in 2% dimethyl sulfoxide (final concentration, 0.05%).
All data are represented as means ± standard deviation. One-way analysis of variance followed by Dunn (post-hoc) test was performed to compare the differences between drug-treated groups. The number of preparations sampled from separate animals is indicated by n. A P-value of <0.05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA).
The changes in the body weight changes were calculated by subtracting the values at the beginning of TNBS-colitis induction (day 0) from that on the day of sacrifice (day 3) (Fig. 1A). A slight increase in body weight was observed in the normal and ethanol-treated groups. Compared to that in the normal group, the mean body weight of the TNBS-colitis group was significantly decreased (+2.3 g vs. -0.85 g, P < 0.05). Compared to that in the TNBS-colitis group, a significant body weight gain was noted in the PRE084 treatment group (1.2 ± 0.2 g, P = 0.05) (Fig. 1A). The weight loss in the TNBS group, which was partially recovered by PRE084 pretreatment, returned to almost the original level after the BD1047 + PRE084 co-treatment (2.1 ± 0.3 g, P = 0.05) (Fig. 1A).
The macroscopic score was significantly higher in the TNBS-colitis group than in the ethanol group, significantly decreased after PRE084 pretreatment, and was restored to the original level by co-treatment with BD1047 and PRE084 (Fig. 1B).
In the histological analysis, the colon section of the normal (control) group exhibited a typical structure with normal submucosal and muscular layers and a regular crypt structure of the mucosa (Fig. 2A). Treatment with 50% ethanol only slightly damaged the intestinal mucosa (Fig. 2B). In contrast, in the TNBS-treated group, inflammation caused local loss of the crypt and superficial epithelium, along with epithelial cell loss in the crypt area reaching the submucosal layer, edema, and severe hemorrhage of the intestinal mucosa. The superficial area was extensively damaged, and an enlarged colon was observed (Fig. 2C). PRE084 pretreatment significantly reduced TNBS-induced damage to the intestinal mucosa, and epithelium partially recovered from the loss of crypts and inflammation (Fig. 2D). Co-treatment with BD1047 and PRE084 attenuated the protective effect of PRE084, and the level of damage was similar to that of the TNBS-treated group (Fig. 2E).
As shown in Fig. 3, the levels of TNF-α, IL-1β, and IL-6 in the TNBS group were significantly higher than those in the control or 50% ethanol groups. Furthermore, in the group co-treated with PRE084 and BD1047, cytokine levels were similar to those in the TNBS group. Compared to that in the 50% ethanol group, TNF-α, IL-1β, and IL-6C increased by 2.9-fold, 3.1-fold, and 4.8-fold, respectively, in the TNBS-treated group. TNBS-induced increase in cytokine levels was reduced by 47% for TNF-α, 37% for IL-1β, and 46% for IL-6, after pretreatment with PRE084. In the PRE084 + BD1047 group, the levels of TNF-α, IL-1β, and IL-6 that were reduced by PRE084 were restored to 96%, 105%, and 87% of those of the TNBS group, respectively.
As shown in Fig. 4A and B, the levels of GSH and SOD in the TNBS group were significantly lower than those in the control or 50% ethanol groups. However, MPO activity was significantly higher in the TNBS group (Fig. 4C). Compared to that in the 50% ethanol group, GSH levels decreased from 24.3 ± 4.1 U/mg to 15.6 ± 2.8 U/mg protein (P < 0.05), and SOD levels decreased from 15.3 ± 2.4 U/mg to 6.5 ± 1.1 U/mg protein (P < 0.05) in the TNBS-treated group. GSH level which was significantly decreased in the TNBS group were partially restored in the PRE084 group (21.8 ± 4.4), but decreased again in the PRE084 + BD1047 group to levels similar to those in the TNBS group (17.4 ± 3.7), though it was not significant. The SOD level decreased in the TNBS group was significantly restored in the PRE084 group (12.4 ± 1.9, P < 0.05), and was again reduced in the PRE084 + BD1047 group (7.7 ± 1.1, P < 0.05). MPO activity was used as a measure of TNBS-induced inflammation. Compared to that in the 50% ethanol group, the MPO activity in the TNBS group was significantly higher (5.7 ± 1.0 U/mg protein vs. 33.6 ± 6.2 U/mg protein, P < 0.05) (Fig. 4C). Compared to that in the TNBS group, the PRE084 group had a significantly lower MPO activity of 20.4 4.1 U/mg protein (P < 0.05), which was restored to 35.9 ± 7.3 (P < 0.05) in the PRE084 + BD1047 group.
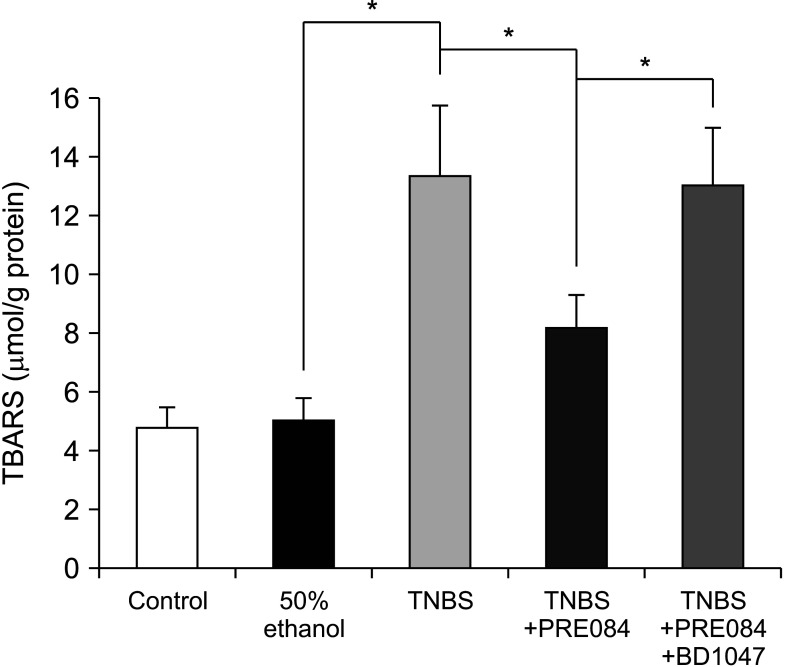
The TBARS levels of the colon homogenates were measured to obtain an estimate of lipid peroxidation, which is known to be associated with UC. As shown in Fig. 5, TNBS treatment significantly increased the production of TBARS compared to that in the control group (4.85 ± 0.7 µmol/g to 13.44 ± 2.3 µmol/g protein, P < 0.05). No significant difference in TBARS levels was observed between the control and the 50% ethanol groups. Compared to TBARS levels in the TNBS group, those in the PRE084 group were significantly lower at 8.23 ± 1.1 µM/g protein (P < 0.05), and were significantly restored in the PRE084 + BD1047 group to 13.1 ± 1.2 µmol/g protein (P < 0.05).
Our study suggests that colitis induced by TNBS is significantly inhibited by the sigma 1 receptor agonist PRE084, and this protective effect is significantly reduced by the sigma-1 receptor antagonist BD1047. The major findings of this study are as follows: (1) the UC severity induced with intrarectal administration of TNBS was higher than that with ethanol alone; (2) PRE084 significantly reduced the size of UC lesions, the levels of proinflammatory cytokines, MPO and TBARS activities; however, it significantly increased the levels of GSH and SOD; (3) the protective effect of PRE084 was significantly attenuated by co-treatment with BD1047, and the severity of TNBS-induced UC reverted to the original diseased state.
TNBS at concentrations of 50–150 mg/kg has been widely used to induce acute colitis in mice. In a preliminary experiment for selecting the concentration and time for acute mild colitis, 25 mg, 50 mg, and 100 mg/kg of TNBS were applied, and weight loss, severity of inflammation, and mortality rate were investigated after 24, 48, 72, and 120 hours. When 25 mg/kg of TNBS was applied, weight loss and severity of inflammation were rarely observed even after 72 and 120 hours. When 50 mg/kg of TNBS was applied, weight loss and severity of inflammation gradually increased over time, reaching a peak value after 72 hours, and thereafter, there was a tendency to recover. When 100 mg/kg of TNBS was applied, the mortality rate was about 20%, and weight loss and severity of inflammation almost reached a peak after 24 hours, and thereafter, there was a tendency to gradually recover time-dependently. Therefore, in this study, TNBS at a concentration of 50 mg/kg was selected to induce mild acute colitis, and the protective effect of PRE084 was investigated by selecting the time of 72 hours after induction for colitis.
UC is an inflammatory gastrointestinal tract disease with an increasing incidence worldwide. It is characterized by disruption of the epithelial barrier and intestinal homeostasis, tends to progress to malignancy, and poses a serious threat to patients owing to its increased recurrence [7]. The effects of well-established treatments such as steroids, sulfasalazine, and immunosuppressants are unsatisfactory. Various side effects and frequent recurrences even after remission occur during long-term treatment. Therefore, there is a strong demand for developing effective therapeutic agents for treating UC. Several experimental models have been developed to study UC. Among them, the TNBS-induced UC model is characterized by inflammation of the mucosal epithelium, and histopathological and immunological changes in it are similar to those of human UC. Since the model has excellent reproducibility and reliability, it was used in this study to evaluate the protective action of sigma-1 receptors. Therefore, the protective effect of PRE084 and the inhibitory effect of BD1047 were evaluated using the TNBS-induced colitis model in this study.
Sigma-1 receptors act as chaperones, and in response to ligand binding, they activate intracellular ion channels and protein kinases in neurodegenerative diseases, thereby increasing antioxidant activity and alleviating inflammation, leading to neuroprotective effects [1920]. Sigma-1 receptors are mainly distributed in the mucosa and submucosal plexus of the intestinal tract. An activated sigma-1 receptor exerts a protective effect on the gastrointestinal mucosa by increasing alkaline secretion [2324]. Sigma-1 receptor agonists have been used to relieve enteritis-associated abdominal pain and have recently been used to alleviate abdominal pain in patients with cholera [25]. Therefore, activating the sigma-1 receptor in the UC model shows an anti-inflammatory effect through antioxidant activity, suggesting a new therapeutic avenue for UC; however, the mechanism of action is still unknown. In the macroscopic and histological analyses in the present study, administration of TNBS to induce UC resulted in significant weight loss, increased disease activity index (DAI), intestinal bleeding, mucosal edema, epithelial cell loss, and inflammatory cell infiltration. Furthermore, weight loss, increase in DAI, UC size, and degree of mucosal damage were significantly reduced by PRE084 pretreatment, and this reduction was reversed by co-treatment with BD1047. These results are consistent with those of other studies showing that sigma-1 receptor activation significantly reduced TNBS-induced inflammation. This protective effect was inhibited by co-treatment with BD1063 and fluvoxamine [2126].
Cytokines are produced by gut-associated immune cells and regulate acute and chronic inflammatory responses at both local and systemic levels as well as the intestinal mucosal barrier. In a previous study using TNBS-induced UC, activated macrophages accumulated in the colonic mucosa, promoting the overproduction of many proinflammatory cytokines such as TNF-α, IL-1β, and IL-6, and inhibiting the production of the anti-inflammatory cytokine IL-10, leading to mucosal damage and ulceration [910]. The severity of colitis is positively correlated with the levels of cytokines in patients with UC [27]. In the present study, the levels of TNF-α, IL-1β, and IL-6 in the TNBS group were significantly higher compared to those in the control and 50% ethanol groups. The colitis-inducing effect of TNBS was significantly reduced by PRE084 pretreatment, and the colitis-inhibitory effect of PRE084 was mostly attenuated by co-administration with BD1047. These results are consistent with the finding that sigma-1 receptor activation reduces the production of TNF-α, IL-1β, and IL-6, thereby significantly reducing inflammation and sepsis caused by TNBS. This strongly suggests that sigma-1 receptor activation could be used to ameliorate the cytokine storm observed in patients with coronavirus disease 2019 [25].
Oxidative stress, caused by excessive production of free radicals and reactive metabolites such as superoxide, nitric oxide, hydroxyl radical, and hydrogen peroxide, is one of the leading causes of many digestive diseases, including ulcers and UC. Currently, the TBARS levels, an indicator of cellular oxidative damage, increase when the levels of endogenous antioxidants such as GSH, SOD, and catalase are significantly reduced, and the oxidation of lipids and proteins is induced by submucosal infiltration of neutrophils. Inflamed colonic mucosa promotes oxidative stress through the generation of ROS, which in turn induces lipid peroxidation, resulting in neutrophil infiltration and a substantial increase in MPO activity. This phenomenon has a significant effect on tissue damage and mucosal dysfunction [28]. Neutrophil infiltration is one of the most prominent histological features of inflamed colonic tissue, and MPO catalyzes the formation of cytotoxic oxidizing agents such as hydrogen peroxide. In addition, a correlation reportedly exists between increased ROS production and disease activity at the site of inflammation in patients with UC. UC has been shown to be inhibited by a drug that prevents ROS production [12]. In this study, TNBS significantly decreased the levels of GSH and SOD compared to those in the control group. Pretreatment with PRE084 remarkably reversed this decrease, and BD1047, in turn, attenuated the effect of PRE084. GSH and SOD are potent antioxidants that protect mucous membranes from oxidative stress. The results of our study are consistent with those of a previous study showing that sigma-1 receptor agonist administration reduces oxidative stress by increasing the production of GSH and SOD, thereby inhibiting UC, and that this antioxidant effect is reduced by sigma-1 receptor antagonists [2021]. These results show that PRE084 inhibits TNBS-induced oxidative stress in mice.
UC sharply increases ROS levels, which aggravates the damage to colonic tissue and significantly increases the levels of lipid peroxidation products. In this study, we monitored MPO activity mainly found in neutrophils and indicated inflammatory cell infiltration and tissue damage as an indicator of ROS levels. Neutrophil infiltration into the inflamed mucosa is one of the features of UC and is characterized by an increase in MPO activity in the inflamed tissue. Peroxidation of lipids in the cell membrane because of oxidative stress destroys the integrity of the intestinal mucosal barrier and activates cytokines, increasing TBARS levels in the intestinal mucosa [29]. In the present study, TNBS treatment significantly increased MPO and TBARS activities compared to those in the control group. PRE084 pretreatment significantly inhibited this effect of TNBS, and the inhibitory effect of PRE084 was in turn attenuated by treatment with BD1047. The reduction of colonic MPO and TBARS levels and absence of cellular infiltration by PRE084 pretreatment indicated that PRE084 has antioxidant and anti-inflammatory properties that can contribute to the prevention of TNBS-induced colitis through the activation of sigma-1 receptors in colonic tissue.
In summary, the present study revealed that PRE084 significantly reduces NBS-induced colitis in mice which is caused by an increase in the protective mechanism of the colon mucosa. Activating the sigma-1 receptor by PRE084 reduces the levels of proinflammatory cytokines, improves the functions of antioxidant and anti-lipid peroxidation factors, and relieves colitis symptoms by restoring the function of the intestinal mucosal barrier. The findings of this in vitro study might therefore serve as a basis and reference for future clinical research.
References
1. Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007; 369:1641–1657. PMID: 17499606.

2. Pithadia AB, Jain S. Treatment of inflammatory bowel disease (IBD). Pharmacol Rep. 2011; 63:629–642. PMID: 21857074.

3. Burisch J, Munkholm P. Inflammatory bowel disease epidemiology. Curr Opin Gastroenterol. 2013; 29:357–362. PMID: 23695429.

4. Kwak MS, Cha JM, Lee HH, Choi YS, Seo SI, Ko KJ, et al. Emerging trends of inflammatory bowel disease in South Korea: a nationwide population-based study. J Gastroenterol Hepatol. 2019; 34:1018–1026. PMID: 30447025.

5. Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012; 380:1606–1619. PMID: 22914296.

6. Ahluwalia B, Moraes L, Magnusson MK, Öhman L. Immunopathogenesis of inflammatory bowel disease and mechanisms of biological therapies. Scand J Gastroenterol. 2018; 53:379–389. PMID: 29523023.

7. Hirten RP, Sands BE. New Therapeutics for ulcerative colitis. Annu Rev Med. 2021; 72:199–213. PMID: 33502898.

8. Antoniou E, Margonis GA, Angelou A, Pikouli A, Argiri P, Karavokyros I, et al. The TNBS-induced colitis animal model: an overview. Ann Med Surg (Lond). 2016; 11:9–15. PMID: 27656280.

9. Kiesler P, Fuss IJ, Strober W. Experimental models of inflammatory bowel diseases. Cell Mol Gastroenterol Hepatol. 2015; 1:154–170. PMID: 26000334.

10. Özden H, Şahin Y, Kilitçi A, Karaca G, Gömeç M, Yildiz A, et al. Comparison of the healing effects of mesazaline and Ganoderma lucidum in acetic acid-induced colitis in rats. Ann Surg Treat Res. 2022; 102:29–35. PMID: 35071117.

11. Nakase H, Sato N, Mizuno N, Ikawa Y. The influence of cytokines on the complex pathology of ulcerative colitis. Autoimmun Rev. 2022; 21:103017. PMID: 34902606.

12. Patlevič P, Vašková J, Švorc P Jr, Vaško L, Švorc P. Reactive oxygen species and antioxidant defense in human gastrointestinal diseases. Integr Med Res. 2016; 5:250–258. PMID: 28462126.

13. Dinallo V, Marafini I, Di Fusco D, Laudisi F, Franzè E, Di Grazia A, et al. Neutrophil extracellular traps sustain inflammatory signals in ulcerative colitis. J Crohns Colitis. 2019; 13:772–784. PMID: 30715224.

14. Pravda J. Hydrogen peroxide and disease: towards a unified system of pathogenesis and therapeutics. Mol Med. 2020; 26:41. PMID: 32380940.

15. Biasi F, Leonarduzzi G, Oteiza PI, Poli G. Inflammatory bowel disease: mechanisms, redox considerations, and therapeutic targets. Antioxid Redox Signal. 2013; 19:1711–1747. PMID: 23305298.

16. Wendland BE, Aghdassi E, Tam C, Carrrier J, Steinhart AH, Wolman SL, et al. Lipid peroxidation and plasma antioxidant micronutrients in Crohn disease. Am J Clin Nutr. 2001; 74:259–264. PMID: 11470730.

17. Santhanam S, Venkatraman A, Ramakrishna BS. Impairment of mitochondrial acetoacetyl CoA thiolase activity in the colonic mucosa of patients with ulcerative colitis. Gut. 2007; 56:1543–1549. PMID: 17483192.

18. Nguyen L, Lucke-Wold BP, Mookerjee S, Kaushal N, Matsumoto RR. Sigma-1 receptors and neurodegenerative diseases: towards a hypothesis of Sigma-1 receptors as amplifiers of neurodegeneration and neuroprotection. Adv Exp Med Biol. 2017; 964:133–152. PMID: 28315269.

19. Gris G, Cobos EJ, Zamanillo D, Portillo-Salido E. Sigma-1 receptor and inflammatory pain. Inflamm Res. 2015; 64:377–381. PMID: 25902777.

20. Pal A, Fontanilla D, Gopalakrishnan A, Chae YK, Markley JL, Ruoho AE. The sigma-1 receptor protects against cellular oxidative stress and activates antioxidant response elements. Eur J Pharmacol. 2012; 682:12–20. PMID: 22381068.

21. Almási N, Török S, Valkusz Z, Tajti M, Csonka Á, Murlasits Z, et al. Sigma-1 receptor engages an anti-inflammatory and antioxidant feedback loop mediated by peroxiredoxin in experimental colitis. Antioxidants (Basel). 2020; 9:1081.

22. Min JK, Lee CH, Jang SE, Park JW, Lim SJ, Kim DH, et al. Amelioration of trinitrobenzene sulfonic acid-induced colitis in mice by liquiritigenin. J Gastroenterol Hepatol. 2015; 30:858–865. PMID: 25311527.

23. Motawe ZY, Abdelmaboud SS, Cuevas J, Breslin JW. PRE-084 as a tool to uncover potential therapeutic applications for selective sigma-1 receptor activation. Int J Biochem Cell Biol. 2020; 126:105803. PMID: 32668330.

24. Hara H, Tanaka K, Harada Y, Sukamoto T. Sigma receptor-mediated effects of a new antiulcer agent, KB-5492, on experimental gastric mucosal lesions and gastric alkaline secretion in rats. J Pharmacol Exp Ther. 1994; 269:799–805. PMID: 8182548.
25. Facente SN, Reiersen AM, Lenze EJ, Boulware DR, Klausner JD. Fluvoxamine for the early treatment of SARS-CoV-2 infection: a review of current evidence. Drugs. 2021; 81:2081–2089. PMID: 34851510.

26. Almási N, Török S, Dvorácskó S, Tömböly C, Csonka Á, Baráth Z, et al. Lessons on the sigma-1 receptor in TNBS-induced rat colitis: modulation of the UCHL-1, IL-6 pathway. Int J Mol Sci. 2020; 21:4046.

27. Friedrich M, Pohin M, Powrie F. Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity. 2019; 50:992–1006. PMID: 30995511.

28. Allahtavakoli M, Jarrott B. Sigma-1 receptor ligand PRE-084 reduced infarct volume, neurological deficits, pro-inflammatory cytokines and enhanced anti-inflammatory cytokines after embolic stroke in rats. Brain Res Bull. 2011; 85:219–224. PMID: 21453760.

29. Mostafa AF, Elalfy MM, Shata A, Elhadidy MG. Prophylactic effect of aquatic extract of stevia on acetic acid induced-ulcerative colitis in male rats: a possible role of Nrf2 and PPARγ. J Basic Clin Physiol Pharmacol. 2020; 32:1093–1104. PMID: 33035185.

Fig. 1
Effects of PRE084 and BD1047 pretreatment on weight gain (A) and macroscopic score (B) of each group of mice in 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis model. PRE084 showed protective effects on weight loss and significant improvement of macroscopic score. Six mice were used in each group. *P < 0.05 significantly different from TNBS-induced colitis group. TNBS, TNBS-induced colitis group; PRE084, PRE084 pretreatment group; PRE084 + BD1047, PRE084 and BD1047 pretreatment group.
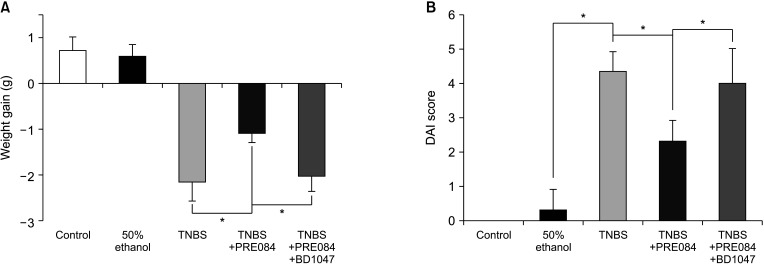
Fig. 2
Effects of PRE084 and BD1047 pretreatment on the microscopic changes of colonic mucosa in mice in TNBS-induced colitis model. Photomicrographs of H&E staining of colonic mucosa (×200). Six mice were used in each group. (A) Control, (B) 50% ethanol, (C) TNBS, (D) PRE084 + TNBS, and (E) PRE084 + BD1047 + TNBS. TNBS-induced colitis group (C) shows severe mucosal layer destruction, infiltration of inflammatory cells, and cryptic damage. PRE084 pretreatment group (D) shows relatively intact surface epithelia and cryptal glands compared with the TNBS-colitis group (C). PRE084 + BD1047 pretreatment group (E) shows the degree of damage to the mucosal layer was similar to that observed in TNBS-induced colitis group (C). TNBS, 2,4,6-trinitrobenzene sulfonic acid.
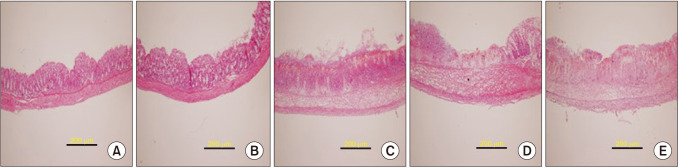
Fig. 3
Effects of PRE084 and BD1047 pretreatment on the levels of TNF-α (A), IL-1β (B), and IL-6 (C) of each group of mice in TNBS-induced colitis model. (A) control, (B) 50% ethanol-treated group, (C) TNBS-induced colitis group, (D) PRE084 + BD1047 pretreatment group, (E) PRE084 + DB1047 pretreatment group, respectively. Six mice were used in each group. Data represent mean ± standard deviation; *P < 0.05 TNBS vs. TNBS + treatment. TNBS, 2,4,6-trinitrobenzene sulfonic acid; TNF, tumor necrosis factor; IL, interleukin.
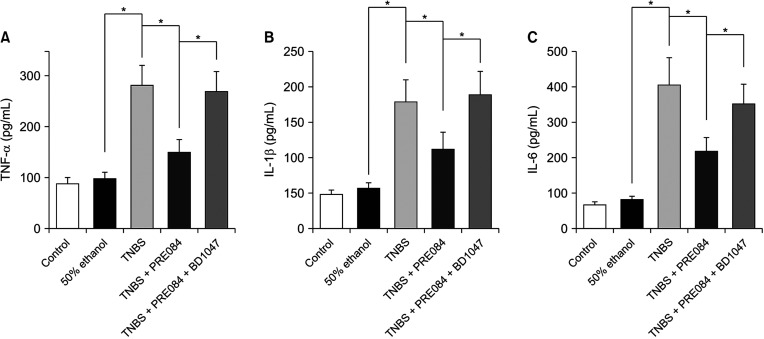
Fig. 4
Effects of PRE084 and BD1047 pretreatment on the levels of glutathione (A), SOD (B), and MPO (C) of each group of mice in TNBS-induced colitis model. (A) control, (B) 50% ethanol-treated group, (C) TNBS-induced colitis group, (D) PRE084 + BD1047 pretreatment group, (E) PRE084+ DB1047 pretreatment group, respectively. Six mice were used in each group. Data represent mean ± standard deviation; *P < 0.05 TNBS vs. TNBS + treatment. TNBS, 2,4,6-trinitrobenzene sulfonic acid; SOD, superoxide dismutase; MPO, myeloperoxidase.
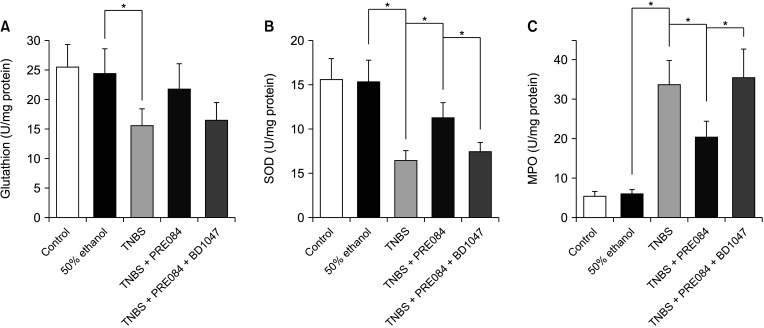
Fig. 5
Effects of PRE084 and BD1047 pretreatment on the levels of TBARS of each group of mice in TNBS-induced colitis model. (A) control, (B) 50% ethanol-treated group, (C) TNBS-induced colitis group, (D) PRE084 + BD1047 pretreatment group, (E) PRE084+ DB1047 pretreatment group, respectively. Six mice were used in each group. Data represent mean ± standard deviation; *P < 0.05 TNBS vs. TNBS + treatment. TBARS, thiobarbituric acid reactive substance; TNBS, 2,4,6-trinitrobenzene sulfonic acid.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download