This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
In patients who have previously undergone subtotal gastrectomy (STG), the remnant stomach is supplied with arterial blood through the splenic artery. It is currently unclear whether the remnant stomach can be safely preserved when performing distal pancreatosplenectomy (DPS) in these patients. Thus, this study aimed to evaluate the safety and feasibility of performing DPS in patients who had undergone a previous STG.
Methods
A multicenter cohort study was performed to identify patients who underwent DPS. Electronic medical data of Clinical Data Warehouse from 7 representative high-volume centers in 5 cities were retrospectively reviewed. A propensity score-matched analysis was performed to match patients who had no history of upper abdominal surgery with patients who had undergone a previous STG.
Results
Fourteen DPS patients who had a history of STG (STG group) were studied and matched to 70 patients who underwent DPS without any history of upper abdominal surgery (non-STG group). All patients in the STG group had the remnant stomach preserved. In most patients, the blood vessel supplying blood to the remnant stomach was the left inferior phrenic artery. There was no significant difference in the incidence of stomach-related complications or length of hospital stay between the 2 groups.
Conclusion
Our study results suggest that the remnant stomach could be safely preserved when performing DPS in patients with a prior STG. However, it is necessary to carefully evaluate the vascular structure of the remnant stomach through preoperative imaging study and closely observe changes to the blue stomach during the operation.
Go to :

Keywords: Distal pancreatectomy, Gastrectomy, Short gastric artery, Splenic artery
INTRODUCTION
Distal subtotal gastrectomy (STG) is a mainstay of treatment for middle and distal-third gastric cancer [
1]. Distal pancreatectomy (DP) with or without splenectomy is currently the gold standard surgical approach for benign and malignant tumors located in the body and tail of the pancreas [
2]. When implementing DP, splenectomy is often accompanied by lymph node dissection around the spleen in malignant diseases. Even for benign tumors in the pancreas, concomitant splenectomy is often forced to perform together because of the close anatomical relationship between the pancreas and splenic vessels.
When looking at blood vessels ligated during the procedure of STG, the left gastric artery (LGA), right gastric artery, right gastroepiploic artery, and left gastroepiploic artery are ligated. Therefore, the main blood supply of the remnant stomach after STG is through arterial branches from the splenic artery (e.g., short gastric artery) [
3]. For the reasons mentioned above, in the case of DPS, the remnant stomach is removed together after dividing the splenic artery and veins [
4].
At present, STG and distal pancreatosplenectomy (DPS) are relatively safe procedures with mortality rates below 3% and 1%, respectively, when performed in isolation [
56]. However, the prevalence of gastric cancer in Korea is relatively high, and long-term survivors who have undergone STG are increasing [
7]. In addition, some pancreatic diseases such as intraductal papillary mucinous neoplasm (IPMN) and pancreatic cancer that require surgery are gradually rising in incidence in these elderly patients. Several authors have reported that patients with a prior STG are at high risk for developing pancreatic cancer, especially with a prolonged post-STG interval [
8910]. Thus, the number of patients requiring DPS with a history of the previous STG increases these days. They could have a risk of loss or reduction of blood flow to the remnant stomach, which may theoretically lead to postoperative ischemic complications of the remnant stomach due to ligation of splenic vessels during the operation. Therefore, in these patients, the surgeon faces a difficult decision to switch from STG state to total gastrectomy or perform spleen preserving DP to preserve splenic vessels and short gastric vessels as much as possible.
A minimal number of retrospective studies or case reports have addressed the safety of the remnant stomach after DPS. Thus, this multicenter study aimed to evaluate clinical outcomes and safety of DPS in patients with a remnant stomach who had a history of the previous STG.
Go to :

METHODS
Study design and patient selection
All patients who underwent DPS for benign or malignant pancreatic tumors during the study period from January 2018 to December 2020 in 7 hospitals of the Catholic Medical Center were comprehensively reviewed from a prospective database. The involved seven affiliated hospitals in the Catholic Medical Center were: Seoul St. Mary’s Hospital, Seoul, Korea; Incheon St. Mary’s Hospital, Incheon, Korea; Bucheon St. Mary’s Hospital, Bucheon, Korea; Uijeongbu St. Mary’s Hospital, Uijeongbu, Korea; St. Vincent’s Hospital, Suwon, Korea; Yeouido St. Mary’s Hospital, Seoul, Korea; and Eunpyeong St. Mary’s Hospital, Seoul, Korea.
Data sources
We used electronic medical records housed in the Clinical Data Warehouse (CDW) of the medical center to identify potentially eligible patients. The CDW includes demographics, diagnoses, doctors’ orders, medications and procedures, laboratory data, and medical imaging data. This study was conducted after obtaining approval from the Institutional Review Board of the Catholic Medical Center (No. XC21WIDI0053). After institutional review boards of all 7 participating hospitals approved the protocol for retrospective review of clinical records, each hospital provided data as specified by a menu-driven database file prepared with instructions for coding fields and drop-down lists where appropriate. Then, using an electronic case report form, all relevant anonymized clinical records were entered into a common database.
Data collection
Demographic data obtained for each patient comprised the following: age, sex, body mass index (BMI), Charlson Comorbidity Index (CCI), American Society of Anesthesiologists (ASA) physical status (PS) classification, drinking and smoking habit, diabetes mellitus (DM), and physioimmunological values (e.g., the Controlling Nutritional Status [CONUT] score, neutrophil-to-lymphocyte ratio [NLR], platelet-to-lymphocyte ratio [PLR], serum level of total protein and albumin). Perioperative variables were operation time, estimated blood loss (EBL), the incidence of transfusion, and combined other organ resection except for pancreas and spleen, pathologic results, the incidence of R0 resection, intensive care unit (ICU) stay, and length of hospital stay. Stomach-related complications were defined as any complications related to the stomach (e.g., delayed gastric emptying, gastric ischemia, peptic ulcer disease, and Mallory-Weiss syndrome). Surgical complications were determined from patient records during hospitalization and within 90 days after surgery. They were then stratified according to the grading scheme proposed by the International Study Group of Pancreatic Surgery (ISGPS) and the Clavien-Dindo classification [
11]. We also assessed pre- and postoperative CT images of all patients participating in this study with a radiologist to closely examine their blood flow and vascular distribution in the remnant stomach.
A propensity score-matched analysis was performed to match the STG group (patients with a history of previous STG who underwent DPS) with the non-STG group (patients who underwent DPS during the same period without a previous upper abdominal surgical history, including STG) according to sex, age, BMI, ASA PS classification, CCI, drinking and smoking habit, and physioimmunological values.
Statistical analyses
Continuous variables are expressed as mean ± standard deviation. Categorical variables are presented as number (%). All statistical analyses were performed using the Mann-Whitney U-test for continuous variables and Fisher exact test for categorical variables. The propensity score was calculated using a logistic regression model, including preoperative variables considered to be directly associated with complicated outcomes. The STG group and the control group underwent a 1:5 nearest-available matching of the logit of the propensity score with a caliper width of 0.20 of the standard deviation of the score. All statistically significant preoperative and perioperative variables were included to establish the model. All statistical analyses were conducted using IBM SPSS Statistics ver. 22.0 (IBM Corp., Armonk, NY, USA) and R software (version 3.5.1, R Foundation for Statistical Computing, Vienna, Austria). All comparisons were 2-sided and a P-value of <0.05 was considered to be significant.
Go to :

RESULTS
Study subjects
A cohort of 342 patients who underwent DPS from 7 hospitals representing each country region was obtained. Of these patients, only 221 met the inclusion criteria with all data required for this study, including 14 patients in the STG group and 207 patients in the non-STG group. Baseline characteristics of these patients are shown in
Table 1.
Table 1
Patient characteristics of the STG group and the non-STG group
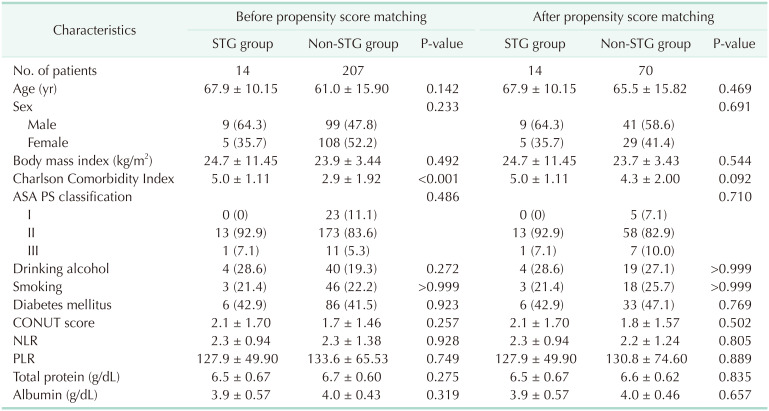

The mean patient age was 67.9 years in the STG group and 61.0 years in the non-STG group. Minimally invasive (MIS) surgery was tried in 1 patient (7.1%) in the STG group and 119 (57.5%) in the non-STG group. Before matching, the CCI of the STG group was higher than that of the non-STG group (mean, 5 ± 1.11 vs. 2.9 ± 1.92; P < 0.001). There was no significant difference in age, ASA PS classification, past medical history of drinking, alcohol, or DM between the STG and non-STG groups. Furthermore, there was no significant difference between the 2 groups in preoperative physiobiological statuses such as CONUT score, NLR, PLR, serum total protein level, or albumin level. After 1:5 propensity score matching, 14 patients in the STG group were matched to 70 similar patients in the non-STG group.
Perioperative outcomes
There was no significant difference in operation time, EBL, transfusion rate, or resection rate for other organs between the 2 groups. We attempted a laparoscopic approach in 5 patients with a STG history. However, due to severe adhesion of previous surgery, most cases were converted to open surgery during MIS. Only one case could be performed without open conversion. In the non-STG group, MIS was performed in 40 patients (57.1%). Pathologically, the proportion of patients with the malignant disease was not significantly different between the 2 groups. Surgical outcomes are presented in
Table 2.
Table 2
Surgical outcomes of the STG group and the non-STG group


Safety of the remnant stomach
Stomach-related complications (gastric necrosis/perforation, ischemic gastritis, or delayed gastric emptying) did not occur in the STG group. One patient in the non-STG group (1.4%) suffered delayed gastric emptying. However, there was no statistically significant difference in the rate of stomach-related complications between the 2 groups (P > 0.999). The time to resume the oral soft diet after the operation was similar for the 2 groups (5.9 days vs. 4.7 days, P = 0.138). The time of drain catheter removal in the STG group was not significantly different from that of the non-STG group (P = 0.153).
Postoperative outcomes
There were no significant differences in the incidence of postoperative pancreatic fistula (grade B/C pancreatic fistula rate, 0% in the STG group
vs. 1.4% in the non-STG group; P = 0.866) or the rate of postoperative complication according to the Clavien-Dindo classification (P = 0.440). One patient (7.1%) in the STG group and 3 patients (4.3%) in the non-STG group underwent reoperation. One patient in the STG group had a sepsis-related multiple organ failure. The 3 patients in the non-STG group had small bowel strangulation caused by adhesion and pancreatic fistula-related bleeding. The length of ICU and hospital stay were not significantly different between the 2 groups (
Table 3).
Table 3
Postoperative morbidities in the STG group and the non-STG group after distal pancreatectomy


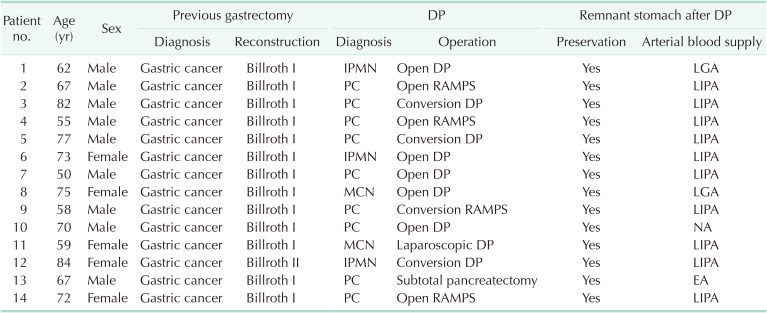
Pathologic and vascular characteristics of the STG group
Characteristics of the primary gastric disease before DPS and pancreatic lesions, along with postoperative investigation of blood supply to the remnant stomach in the STG group, are summarized in
Table 4. All patients were previously diagnosed with gastric cancer. They then underwent DPS for various pancreatic neoplasms, including 9 cases (64.3%) of pancreatic cancer, 3 cases (21.4%) of IPMNs, and 2 cases (14.3%) of mucinous cystic neoplasm. The remnant stomach of all patients was well preserved after DPS. In 10 patients, postoperative enhanced CT revealed blood supply to the remnant stomach from the left inferior phrenic artery (LIPA), 2 from LGA, and 1 from the branch artery of the esophageal artery. However, clear blood vessels through which the blood supply took place could not be identified for 1 patient (
Fig. 1).
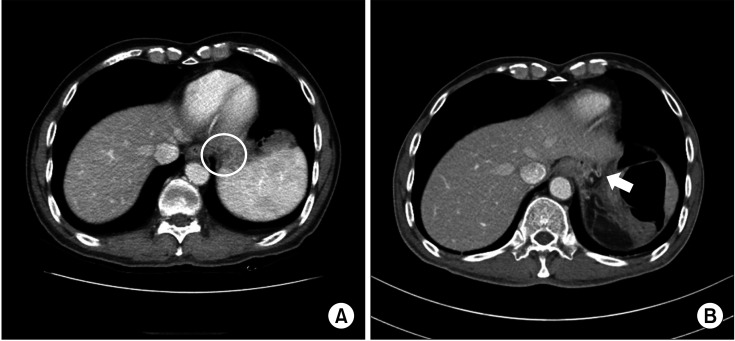 | Fig. 1(A) Preoperative CT for patient 9. (B) Postoperative CT showing the developed left inferior phrenic artery (arrow) in patient 9.
|
Table 4
Detailed characteristics of patients who underwent DP after a precious subtotal gastrectomy

Go to :

DISCUSSION
This cohort study using CDW data found that the remnant stomach was safely preserved when DPS was performed for patients who had previously undergone STG. However, there was a debate about whether preservation of remnant stomach after DPS was possible in patients who had undergone STG. Since the remnant stomach, after STG, received blood supply mainly from the short gastric artery, a branch of the splenic artery, it was hypothesized that the risk of gastric ischemia would increase due to insufficient blood supply to the remnant stomach after DPS. Therefore, surgeons were deeply concerned about whether total gastrectomy should be performed in the STG state. However, Takahashi et al. [
3] and Kimura et al. [
4] have reported preserving remnant stomach is possible and that DPS could be safely committed. On the other hand, a few studies have reported severe complications caused by ischemia of the remnant stomach. Isabella et al. [
12] have reviewed 28 cases of ischemic necrosis of the remnant stomach after STG and found gastric remnant ischemia in 16 cases (57%) after distal gastrectomy with splenectomy. Alshehri et al. [
13] have noted that necrosis of remnant stomach could occur after laparoscopic STG with unplanned splenectomy. Since these studies were just case reports or series with small sample size, they were insufficient to provide reliable evidence. In this study, to collect a more significant number of cases than those in previous studies, we attempted to gather all surgical experiences performed in 7 hospitals of 5 major cities. Stomach-related complications did not occur in the STG group. Furthermore, we found no statistical difference in the time to start oral intake or recovery after surgery between the 2 groups.
Potential elements of successfully preserving the remnant stomach in this patient population include the following. Collateral blood supply via recurrent branches of the LIPA or descending branches of the esophageal artery might have played an essential role in perfusing the remnant stomach. Yovanovitch [
14] has reported that in 81% of a series of 100 gastrectomies, the LIPA supplies 3–4 branches, anastomosing with the short gastric artery (17 cases). And Cate and Dawson [
15] have reported that necrosis and perforation show increased incidence when inferior phrenic arteries are cut in an animal experiment. Blood supply to the remnant stomach was also observed from branches of the LGA in 2 patients. These patients were accompanied by splenectomy due to spleen injury during STG. LGA was preserved for concerns about ischemia or necrosis of the remnant stomach. CT images before and after surgery showed that blood vessels supplying blood to the remnant stomach were naturally expanded after the operation (
Fig. 2).
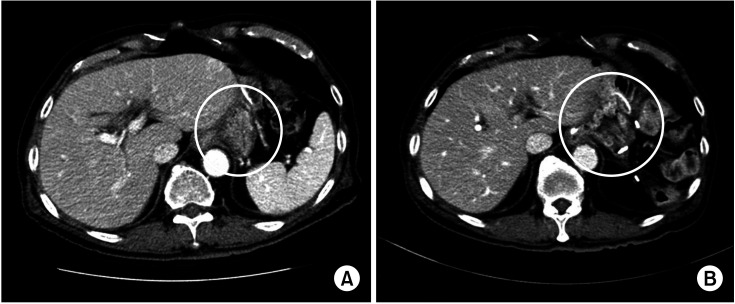 | Fig. 2CT images before (A) and after (B) surgery showed that blood vessels (circles) supplying blood to the remnant stomach were naturally expanded after the operation.
|
Even if there is no precise supply of blood vessels to the remnant stomach in the postoperative image study, it can be estimated that the intramural network of mucosal and submucosal plexus is abundantly developed, resulting in a sufficient blood supply to the remnant stomach [
3]. In addition, when STG is performed for gastric malignancies, the blood requirement is expected to be low due to the small amount of the remnant stomach.
In the present study, the remnant stomach blood supply was not confirmed using angiography CT or intraoperative fluorescence indocyanine green angiography. The indication of DPS in patients after the previous STG was carefully evaluated in a preoperative setting. For example, for patients with comorbidities of circulatory disorders such as congestive heart failure, vasculitis, and uncontrolled DM, the possibility of total gastrectomy was fully considered during DPS because of concerns about an insufficient blood supply to the remnant stomach. While proceeding with DPS, whether a blue stomach occurred was checked over time, and any color change was observed in the remnant stomach. Otherwise, DPS was planned without hesitation when the LGA or LIPA was confirmed on preoperative CT. Lastly, even if there are no comorbidities as mentioned above, we should always keep in mind that ischemia in the remnant stomach could occur during operation. Therefore, total gastrectomy can be considered when there is a blue stomach.
The present study has several limitations. First, this study was retrospective despite its being performed using multicenter, prospectively collected databases. This could lead to some missing data or inevitable bias in the analysis. However, since study subjects were patients with unusual clinical situations, the sample size was naturally small. Our study was the result of reviewing the experiences of all 7 medical institutions. We analyzed a much larger number of patients than in previous studies. Additionally, in an observational study, propensity score matching is used for covariates usually not balanced between groups. Therefore, observed covariates are balanced at each value of the propensity score. We tried to reduce various confounding factors that might affect the study results, thereby improving the validity. Second, because of the lack of blood flow measurements, any modest reduction in blood flow into the remnant stomach would have been difficult to assess on preoperative CT.
Despite these limitations, the current study focuses on an important aspect of surgical treatment for patients with a history of STG, which is the optimal allocation and protocol design of treatment regimens based on patient characteristics. Further investigations with a more significant number of patients are needed to conclusively determine how to manage the remnant stomach when performing DPS in patients with a history of STG.
In conclusion, our study suggests that the remnant stomach could be safely preserved when performing DPS in patients with STG history. First, however, it is necessary to consider whether the remnant stomach could be preserved through preoperative imaging examination and carefully observe whether there is ischemia in the remnant stomach during operation.
Go to :







 PDF
PDF Citation
Citation Print
Print







 XML Download
XML Download