Abstract
Although anti-hepatitis A virus (HAV) IgM non-reactive and anti-HAV total (immunoglobulin [Ig] M and IgG) reactive results are generally interpreted as immunity to HAV, some early acute hepatitis A patients show the same results. We compared IgM detection sensitivity between anti-HAV IgM and anti-HAV total assays. Acute hepatitis A patients’ samples were serially diluted and tested with Elecsys anti-HAV IgM and total assay (Roche Diagnostics). This resulted in anti-HAV IgM non-reactive but anti-HAV total reactive results. Samples of two hepatitis A patients showing false-negative anti-HAV IgM at initial presentation were analyzed with Elecsys, Atellica (Siemens Healthineers), and Alinity (Abbott Laboratories) HAV assays. Elecsys, Atellica, and Alinity anti-HAV IgM converted reactive on hospital day 3, whereas Elecsys and Atellica anti-HAV total results were reactive from hospital day 1. The anti-HAV total assay had higher sensitivity in detecting IgM antibodies than the anti-HAV IgM assay.
Graphical Abstract
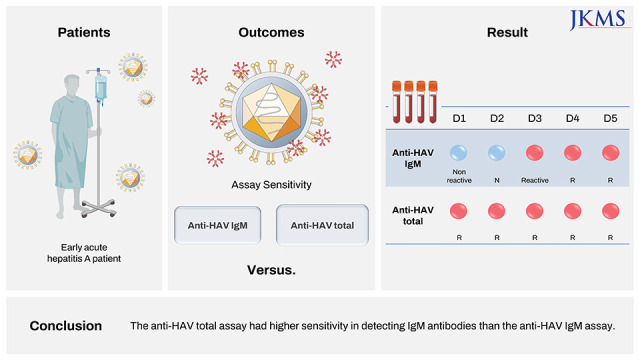
It is estimated that 1.4 million hepatitis A virus (HAV) infection cases occur each year worldwide.1 HAV infection is generally self-limited but can also cause large epidemics caused by close personal contact or contaminated food or water, and less than 1–5% of HAV-infected adults develop fulminant hepatitis.23456 Therefore, prompt diagnosis is important for infection control and patient isolation.
Clinical presentation of hepatitis A overlaps with many other gastrointestinal and febrile conditions.7 Therefore, it cannot be diagnosed solely based on clinical evidence, and the detection of serum anti-HAV immunoglobulin (Ig) M antibodies is needed.
Depending on the HAV serologic assay manufacturer, there is a difference in the type of immunoglobulin detected. Most assays detect IgM and total (IgM and IgG) antibodies, such as Elecsys (Roche Diagnostics, Mannheim, Germany), Atellica (Siemens Healthineers, Munich, Germany), DxI (Beckman Coulter, Brea, CA, USA), Vitros (Ortho Clinical Diagnostics, Pencoed, UK), and Liaison (DiaSorin, Saluggia, Italy). Alinity (Abbott Laboratories, Abbott Park, IL, USA) detects IgM and IgG antibodies separately.
Although anti-HAV IgM antibodies can also be detected by anti-HAV total assay, reports on anti-HAV total results for acute hepatitis A patients are scarce. Recently, we encountered a patient who was anti-HAV IgM non-reactive but anti-HAV total reactive. Acute hepatitis A was suspected but the results obfuscated whether the patient was immunized to HAV or low concentration IgM was only detectable in anti-HAV total assay.
In this study, we compared IgM detection sensitivity between Elecsys anti-HAV IgM and Elecsys anti-HAV total assays. To our knowledge, this is the first study to compare anti-HAV IgM and total results in early acute hepatitis A patients.
We evaluated how low-concentration anti-HAV IgM antibodies affect anti-HAV IgM and total assays. Anti-HAV IgM reactive samples from three acute hepatitis A patients were diluted manually (1:10 through 1:5,120). Diluted samples were simultaneously tested with Elecsys anti-HAV IgM and total assays. This resulted in Elecsys anti-HAV total reactive but Elecsys anti-HAV IgM non-reactive results below certain IgM concentration (Table 1).
Initial anti-HAV IgM results of 1,351 patients from January to December 2021 were reviewed. According to the final diagnosis, assay results were classified into hepatitis A (n = 43) and non-hepatitis A (n = 1,308) (Fig. 1). In acute hepatitis A patients, Elecsys anti-HAV IgM sensitivity was 95.3% (41/43), whereas Elecsys anti-HAV total sensitivity was 100% (43/43). When we compared initial anti-HAV IgM and anti-HAV total results in 43 hepatitis A patients, the anti-HAV total was reactive regardless of anti-HAV IgM cut-off index (COI) (Table 2). In addition, Elecsys anti-HAV IgM specificity, false-positive rate, and false-negative rate were 99.6% (1,303/1,308), 0.4% (5/1,308), and 4.7% (2/43), respectively.
The performance of the Elecsys anti-HAV IgM assay was analyzed by receiver operating characteristic (ROC) curve analysis. When Elecsys anti-HAV IgM COI of 0.55 was applied, the sensitivity and specificity were 100.0% and 98.4%, respectively (Table 3).
Two Elecsys anti-HAV IgM false-negative patients were further evaluated. Case 1 was a 36-year-old man who presented for fever, chills, and dark urine with 3-day onset. He reported that he had raw fish a week ago, and did not receive hepatitis A vaccine. His alanine aminotransferase (ALT) level was 2,699 IU/L (normal < 40 IU/L), aspartate transaminase (AST) level was 2,244 IU/L (normal < 40 IU/L), alkaline phosphatase (ALP) level was 123 IU/L (normal 40–129 IU/L), direct bilirubin level was 2.34 mg/dL (normal < 0.3 mg/dL), C-reactive protein (CRP) level was 6.86 mg/dL (normal < 0.5 mg/dL) on admission. Viral hepatitis was suspected as he had characteristic findings, such as elevated AST and ALT levels (> 1,000 IU/L), elevated serum bilirubin (< 10 mg/dL), and elevated ALP (up to 400 U/L).89 Hepatitis B virus (HBV) and hepatitis C virus (HCV) serologic markers were non-reactive with hospital day 1 sample. For HAV, Elecsys and Alinity assays were performed and anti-HAV IgM was non-reactive on hospital day 1 sample, whereas Elecsys anti-HAV total was reactive (Table 4). Elecsys and Alinity anti-HAV IgM converted reactive on hospital day 3 and hospital day 4, respectively.
Case 2 was a 39-year-old man who presented for fever and abdominal pain starting a day ago. He reported that he did not receive hepatitis A vaccine. On admission, his WBC count was 3.35 109/L (normal 4.0–10.0 109/L), hemoglobin level was 8.2 g/dL (normal 14.0–16.5 g/dL), ALT level was 276 IU/L, AST was 345 IU/L, ALP level was 73 IU/L, direct bilirubin level was 0.59 mg/dL, and CRP level was 7.68 mg/dL. Due to nonspecific findings (lower aminotransferase levels relative to general levels noted in viral hepatitis cases and bicytopenia), various diseases, including autoimmune hepatitis and toxic hepatitis, had to be ruled out. For HBV and HCV, only hepatitis B surface antibody was reactive. The hospital day 1 sample was tested with Elecsys, Atellica, and Alinity anti-HAV IgM, and resulted in non-reactive, equivocal, and non-reactive results, respectively, whereas Elecsys and Atellica anti-HAV total results were reactive (Table 4). On hospital day 3, Elecsys, Atellica, and Alinity anti-HAV IgM converted to reactive. Since Alinity IgG assay results were all non-reactive for both cases, reactive anti-HAV total assay results were assumed to be due to IgM antibodies.
To explore the possibility of an increase in the limit of detection (LoD) and possible false-negative effect of the anti-HAV IgM assay, we performed limit of blank (LoB) and LoD analyses according to CLSI EP17-A2.10 Elecsys anti-HAV IgM assay was performed five days for five replicates using two negative samples and two low level anti-HAV IgM samples, respectively. Analysis was performed on Analyze-it (Analyse-it Software, Leeds, UK). LoB and LoD of anti-HAV IgM were 0.29 COI and 0.39 COI, respectively. Although the manufacturer does not provide LoB and LoD specificity for the Cobas e801 analyzer, it was judged to be satisfactory when compared with existing data.11
In this study, we tried to elucidate the difference in the sensitivity of Elecsys anti-HAV IgM and anti-HAV total assays. Serial dilution of anti-HAV IgM reactive samples revealed Elecsys anti-HAV IgM non-reactive and Elecsys anti-HAV total reactive results below certain IgM concentration. The difference in sensitivity between Elecsys anti-HAV IgM and total assay could be identified.
There were two (4.7%, 2/43) acute HAV patients with false-negative Elecsys anti-HAV IgM at initial presentation. This result corresponds to previous reports wherein 6–10% of acute HAV patients show non-reactive anti-HAV IgM at initial presentation.121314 Elecsys anti-HAV total results of these two patients were all reactive. Two acute hepatitis A patients’ Elecsys, Atellica, and Alinity anti-HAV IgM results converted reactive on hospital day 3, and these assays seem to have similar sensitivity. Anti-HAV total assay had better sensitivity than anti-HAV IgM assay in both Elecsys and Atellica.
The difference between anti-HAV IgM and total assay sensitivity may be due to the assay principle. For Elecsys and Atellica anti-HAV IgM assays, IgM-capture assay is performed using a diluted sample (1:400 for Elecsys and 1:100 for Atellica) to detect IgM antibodies. Currently, standardized anti-HAV IgM assays use IgM antibody capture system and detect antibodies by enzyme-linked chemiluminescence or another nonradioactive method. Due to the sample dilution step of the assay, dilution of circulating antibodies is inevitable, which could result in non-reactive results in the early phase of the disease.15 For the anti-HAV total assay, Elecsys and Atellica use competitive immunoassay to detect both IgG and IgM. The competitive method has the advantage that it can measure at least the amount of analyte to be measured.
Generally anti-HAV IgM non-reactive and anti-HAV total reactive results were interpreted as immunity to HAV. In this study, the difference in sensitivity resulted in reactive anti-HAV total but non-reactive anti-HAV IgM assay results in early acute hepatitis A patients, which can lead to a misdiagnosis, unnecessary testing, and delay in isolation of the patient. Clinicians should be aware that non-reactive anti-HAV IgM and reactive anti-HAV total results not only indicate protective immunity to HAV but may also appear in the early acute phase of HAV infection. When Elecsys anti-HAV IgM COI of 0.55 could be applied in hepatitis A suspected cases, and the sensitivity could be increased to 100%. In addition, a follow-up anti-HAV IgM assay could be helpful since the initial non-reactive anti-HAV IgM result converted reactive after a few days. A previous study suggested that non-reactive initial anti-HAV IgM results converted to reactive at least two days after the peak ALT day.12 Although molecular assay can detect viral RNA 14 days before IgM antibody, it is not routinely performed for diagnosis.1617
The limitation of this study is that only a small number of hepatitis A patients were enrolled. However, it is meaningful in that there have been no such reports before.
In conclusion, Elecsys anti-HAV IgM has lower sensitivity than the Elecsys anti-HAV total assay. Low levels of IgM antibodies in early acute hepatitis A patients can show non-reactive anti-HAV IgM and reactive anti-HAV total results. In hepatitis A suspected cases, a lower cutoff of Elecsys anti-HAV IgM COI 0.55 may increase diagnostic sensitivity, and a follow-up anti-HAV IgM assay would be helpful.
References
1. Wang Z, Chen Y, Xie S, Lv H. Changing epidemiological characteristics of hepatitis A in Zhejiang province, China: increased susceptibility in adults. PLoS One. 2016; 11(4):e0153804. PMID: 27093614.

2. Martin A, Lemon SM. Hepatitis A virus: from discovery to vaccines. Hepatology. 2006; 43(2):Suppl 1. S164–S172. PMID: 16447259.

3. Lee WM, Squires RH Jr, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: Summary of a workshop. Hepatology. 2008; 47(4):1401–1415. PMID: 18318440.

4. Kang SH, Kim MY, Baik SK. Perspectives on acute hepatitis A control in Korea. J Korean Med Sci. 2019; 34(36):e230. PMID: 31538417.

5. Kwon SY, Park SH, Yeon JE, Jeong SH, Kwon OS, Lee JW, et al. Clinical characteristics and outcomes of acute hepatitis a in Korea: a nationwide multicenter study. J Korean Med Sci. 2014; 29(2):248–253. PMID: 24550653.

6. Seo JY, Choi BY, Ki M, Jang HL, Park HS, Son HJ, et al. Risk factors for acute hepatitis A infection in Korea in 2007 and 2009: a case-control study. J Korean Med Sci. 2013; 28(6):908–914. PMID: 23772157.

7. Matheny SC, Kingery JE. Hepatitis A. Am Fam Physician. 2012; 86(11):1027–1034. PMID: 23198670.
8. Newsome PN, Cramb R, Davison SM, Dillon JF, Foulerton M, Godfrey EM, et al. Guidelines on the management of abnormal liver blood tests. Gut. 2018; 67(1):6–19. PMID: 29122851.

9. Tong MJ, el-Farra NS, Grew MI. Clinical manifestations of hepatitis A: recent experience in a community teaching hospital. J Infect Dis. 1995; 171(Suppl 1):S15–S18. PMID: 7876641.

10. Clinical and Laboratory Standards Institute (CLSI). Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures. CLSI Document EP17-A2. Wayne, PA: CLSI;2012.
11. 510(k) Substantial equivalence determination decision summary. Accessed February 8, 2022.
https://www.accessdata.fda.gov/cdrh_docs/reviews/k093955.pdf
.
12. Hyun JJ, Seo YS, An H, Yim SY, Seo MH, Kim HS, et al. Optimal time for repeating the IgM anti-hepatitis A virus antibody test in acute hepatitis A patients with a negative initial test. Korean J Hepatol. 2012; 18(1):56–62. PMID: 22511904.

13. Shin HP, Lee JI, Jung SW, Cha JM, Joo KR, Kang SY. Factors for predicting positive results for anti-HAV IgM retesting among initially seronegative patients. Dig Dis Sci. 2010; 55(12):3537–3540. PMID: 20108041.

14. Lee HK, Kim KA, Lee JS, Kim NH, Bae WK, Song TJ. Window period of anti-hepatitis A virus immunoglobulin M antibodies in diagnosing acute hepatitis A. Eur J Gastroenterol Hepatol. 2013; 25(6):665–668. PMID: 23325281.

15. Pondé RA. The serological markers of acute infection with hepatitis A, B, C, D, E and G viruses revisited. Arch Virol. 2017; 162(12):3587–3602. PMID: 28884240.

16. Pisano MB, Giadans CG, Flichman DM, Ré VE, Preciado MV, Valva P. Viral hepatitis update: progress and perspectives. World J Gastroenterol. 2021; 27(26):4018–4044. PMID: 34326611.

17. Bower WA, Nainan OV, Han X, Margolis HS. Duration of viremia in hepatitis A virus infection. J Infect Dis. 2000; 182(1):12–17. PMID: 10882576.

Fig. 1
Patient and data selection flowchart. Elecsys anti-HAV IgM results were classified according to the final diagnosis.
HAV = hepatitis A virus, Ig = immunoglobulin.
Table 1
Elecsys anti-HAV IgM and Elecsys anti-HAV total assay results performed on serially diluted hepatitis A patients’ samples
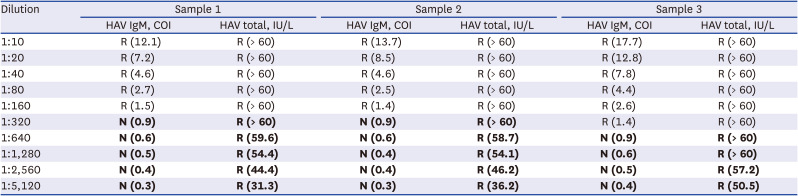
Discrepant anti-HAV IgM and anti-HAV total results were presented as bold. Elecsys anti-HAV IgM results are interpreted as non-reactive (COI < 1.0) and reactive (COI ≥ 1.0). Elecsys anti-HAV total results are interpreted as non-reactive (< 18.0 IU/L), borderline (18.0 ≤ IU/L < 22.0), and reactive (≥ 22.0 IU/L).
HAV = hepatitis A virus, Ig = immunoglobulin, COI = cut-off index, N = non-reactive, R = reactive.
Table 2
Comparison of anti-HAV IgM and anti-HAV total in hepatitis A patients at initial visit to the hospital
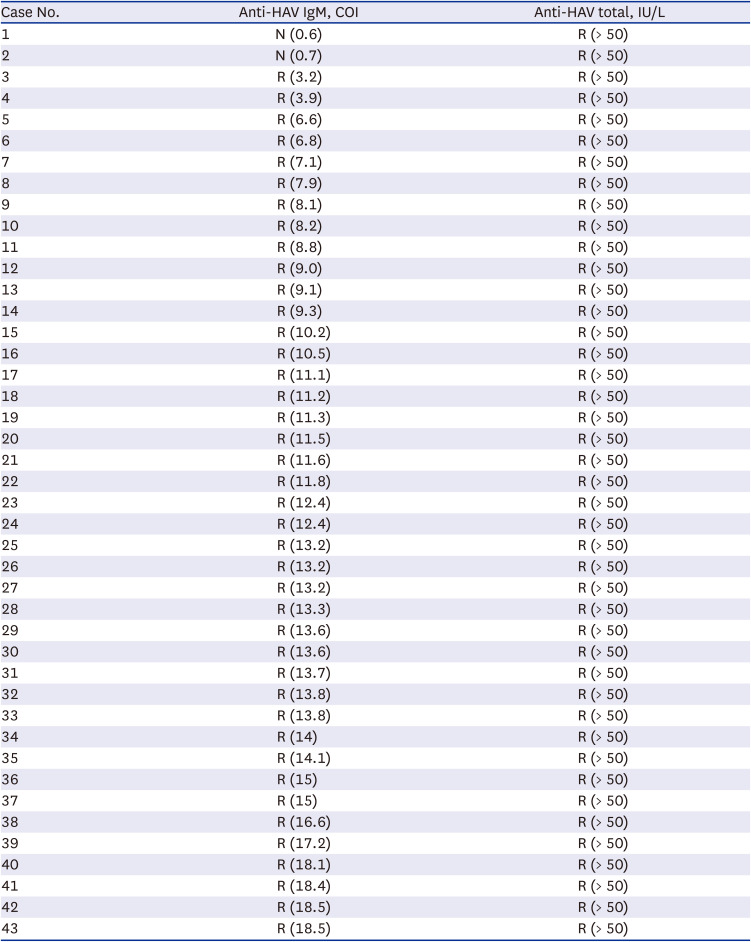
Table 3
Sensitivity and specificity of the Elecsys anti-HAV IgM assay according to the COI
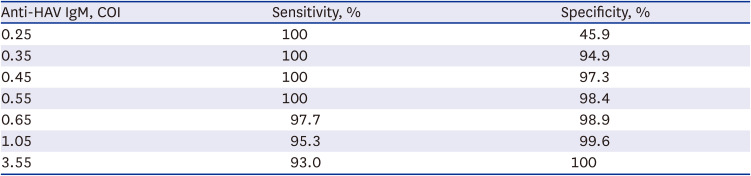
| Anti-HAV IgM, COI | Sensitivity, % | Specificity, % |
|---|---|---|
| 0.25 | 100 | 45.9 |
| 0.35 | 100 | 94.9 |
| 0.45 | 100 | 97.3 |
| 0.55 | 100 | 98.4 |
| 0.65 | 97.7 | 98.9 |
| 1.05 | 95.3 | 99.6 |
| 3.55 | 93.0 | 100 |
Table 4
Serial assay results in initial anti-HAV IgM non-reactive and anti-HAV total reactive hepatitis A cases
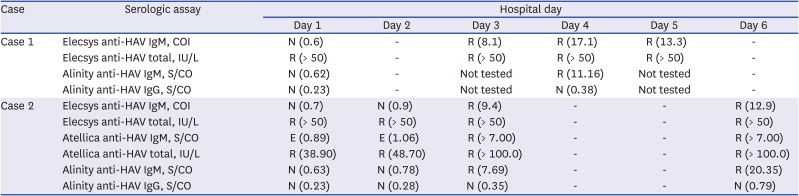
Elecsys anti-HAV IgM results are interpreted as non-reactive (COI < 1.0) and reactive (COI ≥ 1.0). Elecsys anti-HAV total results are interpreted as non-reactive (< 18.0 IU/L), borderline (18.0 ≤ IU/L < 22.0), and reactive (≥ 22.0 IU/L). Alinity anti-HAV IgM results are interpreted as non-reactive (S/CO < 0.80), equivocal (0.80 ≤ S/CO < 1.20), and reactive (S/CO > 1.2). Alinity anti-HAV IgG results are interpreted non-reactive (S/CO < 1.0) and reactive (S/CO ≥ 1.0). Atellica anti-HAV IgM results are interpreted non-reactive (S/CO < 0.80), equivocal (0.80 ≤ S/CO < 1.20), and reactive (S/CO > 1.2). Atellica anti-HAV total results are interpreted as non-reactive (< 20 IU/L) and reactive (≥ 20 IU/L).
HAV = hepatitis A virus, Ig = immunoglobulin, COI = cut-off index, N = non-reactive, R = reactive.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download