This article has been
cited by other articles in ScienceCentral.
Abstract
Post-vaccination myocarditis after administration of the NVX-CoV2373 coronavirus disease 2019 (COVID-19) vaccine has been reported in a limited population. We report the first biopsy-proven case of myopericarditis after administration of second dose of NVX-CoV2373 COVID-19 vaccine (Novavax®) in Korea. A 30-year-old man was referred to emergency department with complaints of chest pain and mild febrile sense for two days. He received the second dose vaccine 17 days ago. Acute myopericarditis by the vaccination was diagnosed by cardiac endomyocardial biopsy. He was treated with corticosteroid 1 mg/kg/day for 5 days and tapered for one week. He successfully recovered and was discharged on the 12th day of hospitalization. The present case suggests acute myopericarditis as a vaccination complication by Novavax® in Korea.
Keywords: Acute Myocarditis, COVID-19 Vaccine, Novavax
INTRODUCTION
The safety of coronavirus 2 (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]) vaccines has emerged as a current global issue, since there had been numerous cases of post-vaccination myocarditis or pericarditis.
12345 Post-vaccination myocarditis after SARS-CoV-2 vaccination have been reported mostly among subjects with messenger RNA (mRNA) coronavirus disease 2019 (COVID-19) vaccine.
67
To date, there have been no post-vaccination myocarditis after administration of the NVX-CoV2373 COVID-19 vaccine (Novavax®) in the literature in Korea. Here we report the first biopsy-proven case of acute myopericarditis associated with NVX-CoV2373 COVID-19 vaccine (Novavax®) administration in Korea.
CASE DESCRIPTION
A 30-year-old man was referred to our emergency department with complaints of chest pain and mild febrile sense for two days. The patient received the first dose of NVX-CoV2373 COVID-19 vaccine (Novavax®) 49 days ago and the second dose 17 days ago. The characteristic of the pain was sharp and as intense as NRS (Numeral Rating Scale) 7, and it was located in the substernal region of the central anterior chest. The pain was worsened by dry cough or deep breathing, and was relieved by rest. The patient had medical history of ulcerative colitis and was taking 1 gram of mesalazine twice a day for five years. The patient denied any recent bowel habit changes including stool frequency and rectal bleeding or use of new medications.
Blood pressure was 110/60 mmHg, heart rate was 104/min, respiratory rate was 18/min, and body temperature was mildly elevated to 37.3°C. There were no detectable abnormalities on physical examination. White blood cells and C-reactive protein level were mildly elevated as 9,300/mL and 1.14 mg/dL. Serum troponin-I level was elevated as 0.645 ng/mL (reference range: 0–0.05 ng/mL) and creatinine kinase-MB type level was also elevated as 6.69 ng/mL (reference range: 0–5 ng/mL). Initial electrocardiography and chest X-ray were unremarkable (
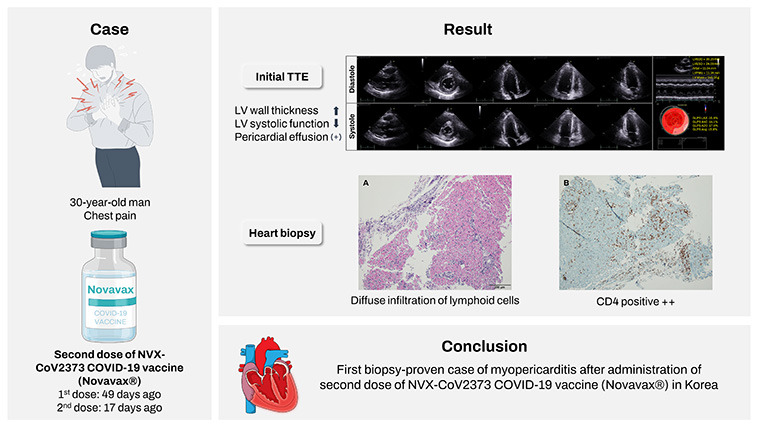
Supplementary Fig. 1). Echocardiography revealed edematous thickening of left ventricular (LV) wall with global hypokinesia and a small amount of pericardial effusion. LV ejection fraction was mildly reduced as 48% by modified Simpson’s method. Right ventricular wall thickness and wall motion were normal (
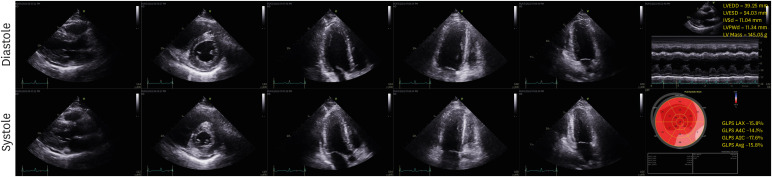
Fig. 1). There was no significant coronary artery stenosis on coronary angiography. With the suspicion of acute myocarditis and pericarditis, subsequent cardiac endomyocardial biopsy (EMB) was performed.
Fig. 1
Initial transthoracic echocardiography. Echocardiographic findings show increased LV wall thickness with mildly reduced LV systolic function and small amount of pericardial effusion.
LV = left ventricular, EDD = end-diastolic dimension, ESD = end-systolic dimension, IVSd = interventricular septum at diastole, PWd = posterior wall thickness at diastole.
Serologic viral markers, including Epstein-Barr virus, parvo B19 virus, cytomegalovirus, and coxsackievirus were negative. Polymerase chain reaction tests on nasal swab for common respiratory viruses, including SARS-CoV-2 were also negative. Antinuclear antibody and rheumatoid factor tests revealed non-specific.
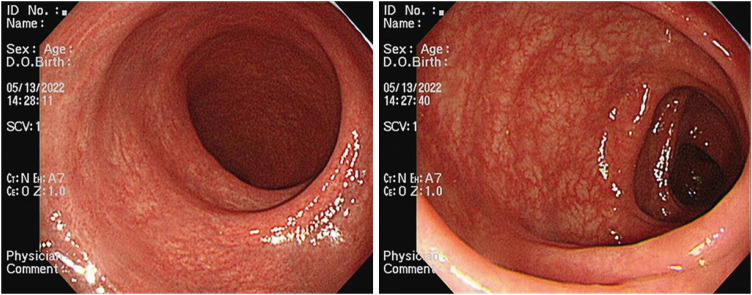
To investigate the disease activity for ulcerative colitis, fecal calprotectin level and Mayo score were checked. Fecal calprotectin level was 75 μg/g, and Mayo endoscopic score was 0–1 point as it showed mild erythema, normal vascular pattern, and no erosion on sigmoidoscopy (
Fig. 2). Therefore, the activity of his ulcerative colitis was considered quiescent.
Fig. 2
Sigmoidoscopic findings. Endoscopic findings show mild erythema, normal vascular pattern and no erosion.
Body temperature was elevated up to 37.8°C, but vital signs were stable during the first 24 hours of hospitalization. A 1 mg/kg/day of intravenous corticosteroids was administered to the patient for 5 days, and then tapered 10 mg per week. Troponin I and C-reactive protein were normalized after hospital day 5 (
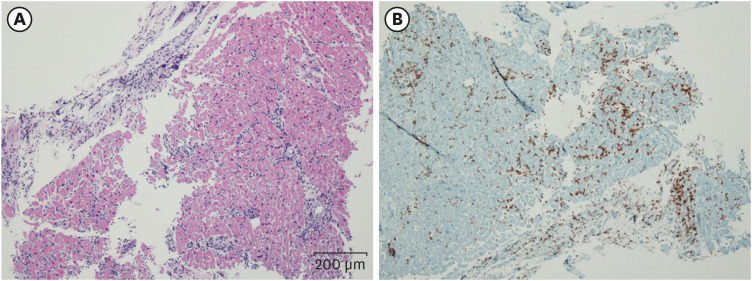
Fig. 3). On histopathology and immunohistochemistry of the patient’s EMB, a diffuse inflammatory infiltration, with CD4+ T lymphocyte predominance, was observed within myocardium (
Fig. 4).
Fig. 3
Hospital course during admission. During hospitalization, serum cardiac troponin I and CRP levels restored to normal range with complete relief of chest pain.
NRS = Numeral Rating Scale, HD = hospital day, CRP = C-reactive protein.
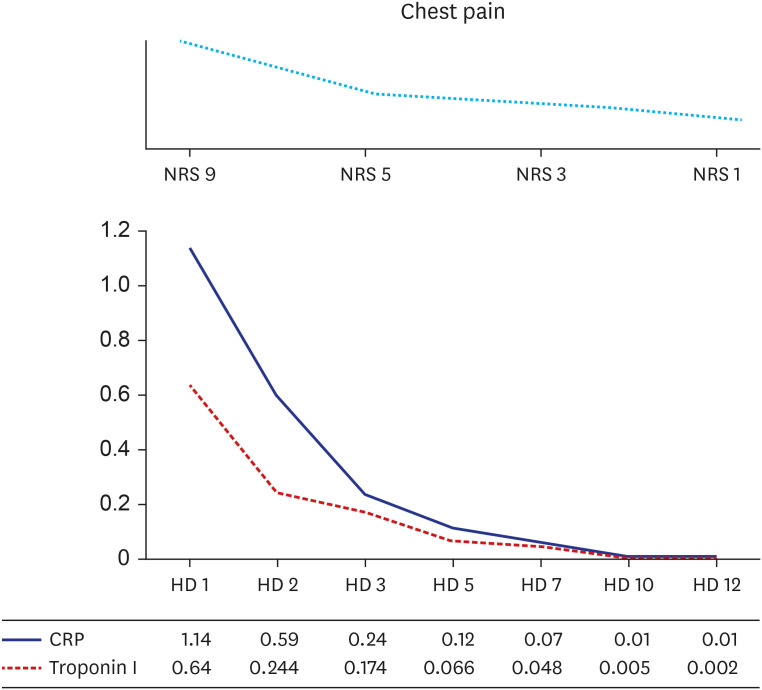
Fig. 4
Histopathologic findings of the heart. (A) HE stains of right ventricle show diffuse infiltration of lymphoid cells within myocardium (HE, ×100). (B) Immunohistochemical stains of right ventricle show CD4 positive cells (×100).
HE = hematoxylin and eosin.
Patient’s chest discomfort remained until hospital day 10, but soon resolved completely. Follow-up echocardiography showed normal LV wall thickness and function and no visualization of previously noted pericardial effusion. The patient was successfully recovered and discharged without complication on the 12th day of hospitalization.
Ethics statement
Informed consent for publication of clinical data was obtained from the patient.
DISCUSSION
We described a case of acute myopericarditis in a 30-year-old man with endoscopic remission of ulcerative colitis, which developed 17 days after the second vaccination of NVX-CoV2373 COVID-19 vaccine (Novavax®).
Recently, cardiac inflammation following SARS-CoV-2 vaccination has been reported, mostly among subjects who were vaccinated with mRNA COVID-19 vaccine.
8910 Meanwhile, a few cases of acute myocarditis after NVX-CoV2373 COVID-19 vaccine have been reported in Korea as well as worldwide. A recent FDA briefing document described five cases of myocarditis occurring after NVX-CoV2373 COVID-19 vaccine and four of them occurred within 20 days after vaccine.
11 In a previous research of a phase 3 trial of NVX-CoV2373 COVID-19 vaccine, only one adverse event of myocarditis was reported among a total of 7593 study population, which occurred 3 days after the second dose of vaccination.
12 It is thought to be a potentially immune-mediated adverse reaction or viral myocarditis.
There are concerns on the increased risk of myocarditis after COVID-19 mRNA vaccination in patients with inflammatory bowel disease (IBD), especially those with immunosuppressive medication. According to the recent large scale clinical study, the incidence of any kinds of adverse events of COVID-19 vaccination in IBD patients were small and similar to that of the general population without IBD.
13 Therefore, the relationship between IBD and the incidence of myocarditis after COVID-19 vaccination are inconclusive and further studies are needed.
The potential mechanisms of vaccine-associated myocarditis or pericarditis are still poorly understood. In a previous study of a phase 1-2 trial of NVX-CoV2373 COVID-19 vaccine, CD4+ T-cell responses were shown as a NVX-CoV2373 induced immunogenicity.
14 In the present case, most of the inflammatory cells found in the EMB specimen were CD4+ T-cell, and thus it is suggested that the immunity of T lymphocyte played an important role in the occurrence of myopericarditis in this case. Further research on the pathophysiologic mechanism of cardiac inflammation after vaccination are needed in the future.








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download